Is Solid To Liquid Endothermic Or Exothermic
Muz Play
Mar 27, 2025 · 5 min read
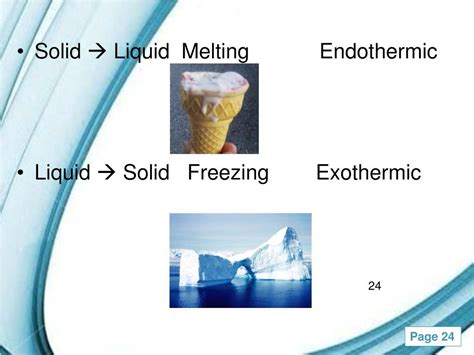
Table of Contents
Is Solid to Liquid Endothermic or Exothermic? Understanding Phase Transitions
The transition of a substance from a solid state to a liquid state, a process commonly known as melting or fusion, is a fundamental concept in chemistry and physics. Understanding whether this phase transition is endothermic or exothermic is crucial for grasping the underlying principles of thermodynamics and the behavior of matter. This comprehensive guide will delve deep into this topic, exploring the intricacies of endothermic and exothermic processes, the role of energy in phase transitions, and the practical applications of this knowledge.
Understanding Endothermic and Exothermic Processes
Before we tackle the specific case of melting, let's establish a clear understanding of endothermic and exothermic reactions. These terms describe the energy exchange between a system and its surroundings during a process.
Endothermic processes absorb energy from their surroundings. This absorption of energy manifests as a decrease in the temperature of the surroundings. Think of it like this: the system "draws" energy from its environment to fuel the process. The enthalpy change (ΔH) for an endothermic process is positive.
Exothermic processes, on the other hand, release energy to their surroundings. This release of energy leads to an increase in the temperature of the surroundings. The system "gives off" energy. The enthalpy change (ΔH) for an exothermic process is negative.
The Solid to Liquid Transition: A Closer Look
Now, let's focus on the solid-to-liquid transition. To understand whether this is endothermic or exothermic, consider what's happening at the molecular level.
In a solid, particles (atoms, ions, or molecules) are tightly packed together in a highly ordered arrangement. Strong intermolecular forces hold these particles in place, restricting their movement.
When a solid is heated, the kinetic energy of its particles increases. This increased kinetic energy overcomes the intermolecular forces holding the particles in their fixed positions. As a result, the particles gain more freedom of movement, transitioning from a rigid, ordered structure to a less ordered, more fluid state – the liquid state.
This process of overcoming intermolecular forces requires energy input. The energy is absorbed from the surroundings, resulting in a decrease in the surrounding temperature. Therefore, the solid to liquid phase transition is an endothermic process.
The Role of Heat in Melting
The heat energy absorbed during melting is used primarily to weaken and eventually break the intermolecular forces holding the solid together. It's not simply increasing the kinetic energy of the particles, although that does happen as well. The energy is directly invested in changing the state of matter.
This absorbed heat energy is also known as the latent heat of fusion. The latent heat of fusion is the amount of heat required to change one unit mass of a substance from a solid to a liquid at its melting point without changing its temperature.
Evidence Supporting the Endothermic Nature of Melting
Several observations support the conclusion that melting is endothermic:
-
Temperature Remains Constant During Melting: If you heat a solid, its temperature will generally rise until it reaches its melting point. At the melting point, the temperature will remain constant despite continued heating. This is because the added energy is being used to break the intermolecular forces, not to increase the kinetic energy (and thus temperature) of the particles.
-
Cooling Effect: If you put ice cubes into a drink, the drink becomes colder. This is because the melting ice absorbs heat energy from the drink, causing the drink's temperature to decrease.
-
Energy Calculations: Using calorimetry, a method for measuring heat flow, the heat absorbed during melting can be quantified and confirmed to be positive.
Practical Applications of Understanding Endothermic Melting
The knowledge that melting is an endothermic process has numerous practical applications:
-
Refrigeration and Air Conditioning: These systems rely on the endothermic nature of phase transitions (like the evaporation of refrigerants) to absorb heat from the surrounding environment, lowering the temperature.
-
Ice Packs: Ice packs used for injuries rely on the endothermic melting of ice to absorb heat, preventing further inflammation.
-
Metallurgy: Understanding the melting points and the energy required for melting is crucial in metallurgy for controlling processes like casting and welding.
-
Food Preparation: Cooking and baking often involve phase transitions; for example, melting butter or chocolate. Understanding the endothermic nature of these processes helps control the cooking process.
-
Material Science: The melting behavior of materials is a critical factor in designing and developing new materials with desired properties.
Exceptions and Considerations
While melting is generally endothermic, there are subtle exceptions and considerations:
-
Pressure Effects: Under certain high-pressure conditions, the melting point of some substances can decrease, leading to a situation where melting might appear less endothermic or even exothermic. However, this is a deviation from the typical behavior and requires very specific conditions.
-
Supercooling: Supercooling is a phenomenon where a liquid can be cooled below its freezing point without solidifying. This can complicate the observation of the endothermic nature of melting.
-
Specific Substances: While the overwhelming majority of substances exhibit endothermic melting, it’s important to note that there are some unusual cases where the process might show slight variations due to unusual intermolecular forces or other complex factors.
Conclusion: Melting as an Endothermic Process
In conclusion, the transition from a solid to a liquid is demonstrably an endothermic process. This is due to the energy required to overcome the intermolecular forces holding the particles in the solid state. This fundamental principle has vast implications in various scientific fields and everyday applications, underscoring the importance of understanding the thermodynamic basis of phase transitions. The absorption of heat during melting is a key characteristic of this process, and its applications range from cooling systems to material science and cooking. While there might be some exceptions under extreme conditions, the general rule remains consistently true: melting requires energy input, solidifying that it's an endothermic change.
Latest Posts
Latest Posts
-
Density Of Water 22 Degrees Celsius
Mar 30, 2025
-
What Does The Nernst Equation Tell Us
Mar 30, 2025
-
What Are The Characteristics Of A Solid
Mar 30, 2025
-
What Is The Difference Between Empirical Formula And Molecular Formula
Mar 30, 2025
-
What Is The Function Of A State
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Is Solid To Liquid Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
