What Are The Characteristics Of A Solid
Muz Play
Mar 30, 2025 · 7 min read
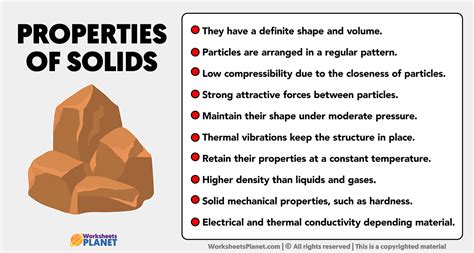
Table of Contents
What are the Characteristics of a Solid?
Solids are one of the four fundamental states of matter, alongside liquids, gases, and plasmas. Understanding the characteristics of solids is crucial in numerous fields, from materials science and engineering to chemistry and physics. This article delves deep into the defining properties of solids, exploring their diverse types and the underlying principles that govern their behavior.
Defining Characteristics of Solids
Solids are characterized by their strong intermolecular forces, resulting in a rigid structure with a definite shape and volume. Unlike liquids and gases, the constituent particles (atoms, molecules, or ions) in a solid are tightly packed and held in relatively fixed positions. This fixed arrangement contributes to their resistance to deformation. Let's explore these key characteristics in more detail:
1. Definite Shape and Volume:
Perhaps the most immediately apparent characteristic of a solid is its fixed shape and volume. You can't easily squish a solid into a different shape, nor can you readily compress it to reduce its volume. This is a direct consequence of the strong attractive forces between the constituent particles, which restrict their movement. Unlike a gas, which expands to fill its container, a solid retains its shape regardless of its container.
2. Strong Intermolecular Forces:
The particles in a solid are held together by strong intermolecular forces, including ionic bonds, covalent bonds, metallic bonds, and van der Waals forces. These forces vary in strength depending on the type of solid, influencing its physical and chemical properties. Stronger bonds lead to higher melting points, greater hardness, and increased resistance to deformation.
3. Rigidity and Incompressibility:
The strong intermolecular forces give solids their rigidity and incompressibility. Rigidity means the solid resists deformation when subjected to external forces. Incompressibility refers to the solid's resistance to changes in volume under pressure. While some degree of compression is possible under extreme conditions, solids are generally much less compressible than liquids or gases.
4. Density:
Solids generally exhibit high density compared to liquids and gases. This is because the constituent particles are closely packed together, leaving little space between them. However, the density of a solid varies depending on the type of solid and its atomic/molecular structure. For example, lead is considerably denser than wood, reflecting differences in atomic packing and atomic mass.
5. Low Kinetic Energy:
The particles in a solid possess relatively low kinetic energy. This means they vibrate around fixed positions but don't have enough energy to overcome the intermolecular forces and move freely like particles in liquids or gases. The amplitude of these vibrations increases with temperature, but the particles remain largely confined to their lattice sites.
6. Crystalline vs. Amorphous Solids:
Solids can be broadly categorized into two types: crystalline and amorphous. This distinction is based on the arrangement of their constituent particles:
Crystalline Solids:
Crystalline solids are characterized by a highly ordered, three-dimensional arrangement of particles. These particles form a repeating pattern called a crystal lattice. This ordered structure gives rise to specific physical properties, including sharp melting points and anisotropic behavior (different properties in different directions). Examples include salt (NaCl), diamond, and quartz.
Amorphous Solids:
Amorphous solids, also known as non-crystalline solids, lack the long-range order found in crystalline solids. Their particles are arranged randomly, like in a liquid, but they retain the rigidity and fixed shape of a solid. They typically exhibit a gradual softening range rather than a sharp melting point. Examples include glass, rubber, and plastics.
Types of Solids Based on Bonding
The type of bonding between the constituent particles plays a significant role in determining the properties of a solid. Several categories exist based on the dominant bonding type:
1. Ionic Solids:
Ionic solids are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). These bonds are strong, resulting in high melting points, hardness, and brittleness. They are typically good insulators in the solid state but can conduct electricity when molten or dissolved in water. Examples include sodium chloride (NaCl) and potassium bromide (KBr).
2. Covalent Solids:
Covalent solids consist of atoms held together by strong covalent bonds. These bonds involve the sharing of electrons between atoms. Covalent solids are generally hard, have high melting points, and are poor conductors of electricity. Diamond is a prime example of a covalent solid, renowned for its exceptional hardness.
3. Metallic Solids:
Metallic solids are characterized by a "sea" of delocalized electrons that move freely among positively charged metal ions. This delocalized electron structure accounts for the high electrical and thermal conductivity of metals. Metallic solids are usually ductile (can be drawn into wires) and malleable (can be hammered into sheets). Examples include iron, copper, and aluminum.
4. Molecular Solids:
Molecular solids consist of molecules held together by relatively weak intermolecular forces, such as van der Waals forces, hydrogen bonds, or dipole-dipole interactions. These forces are weaker than ionic or covalent bonds, leading to lower melting points and greater softness. Many organic compounds exist as molecular solids. Examples include ice (H₂O) and sugar (C₁₂H₂₂O₁₁).
Properties Influenced by Solid Characteristics
The characteristics of solids significantly influence their various properties, making them suitable for diverse applications:
1. Mechanical Properties:
Mechanical properties describe a solid's response to applied forces. These include hardness (resistance to scratching), tensile strength (resistance to stretching), compressive strength (resistance to compression), ductility (ability to be drawn into wires), and malleability (ability to be hammered into sheets). These properties are closely linked to the type and strength of the bonds holding the solid together.
2. Thermal Properties:
Thermal properties encompass a solid's behavior in response to heat. This includes melting point (temperature at which a solid transitions to a liquid), boiling point (temperature at which a liquid transitions to a gas), specific heat capacity (amount of heat required to raise the temperature of a unit mass by one degree), and thermal conductivity (ability to conduct heat). The arrangement and bonding of particles influence these thermal properties.
3. Electrical Properties:
Electrical properties describe a solid's ability to conduct electricity. Electrical conductivity varies significantly depending on the type of solid. Metals are excellent conductors due to their delocalized electrons, while ionic and covalent solids are generally insulators. However, some materials exhibit semiconducting behavior, with conductivity intermediate between conductors and insulators.
4. Optical Properties:
Optical properties describe how a solid interacts with light. These include refractive index (how much light bends when passing through the solid), transparency (ability to transmit light), color (wavelengths of light reflected or absorbed), and luminescence (emission of light after excitation). The crystal structure and electronic configuration influence a solid's optical properties.
Applications Based on Solid Characteristics
The diverse characteristics of solids lead to their widespread use in various applications:
- Construction: Strong and durable solids like concrete, steel, and bricks are essential for building structures.
- Electronics: Semiconductors like silicon and germanium are the foundation of modern electronics.
- Medicine: Biocompatible solids are used for implants, drug delivery systems, and medical devices.
- Energy: Solids play a role in energy storage (batteries) and energy production (solar cells).
- Transportation: Strong and lightweight materials like alloys are used in vehicles and aircraft.
- Packaging: Solids provide protection and containment for various products.
- Jewelry: Precious metals and gemstones are valued for their beauty and durability.
Conclusion
The characteristics of a solid, encompassing its definite shape and volume, strong intermolecular forces, rigidity, and crystalline or amorphous structure, define its diverse properties and applications. Understanding these characteristics is fundamental to materials science, engineering, and various other scientific disciplines. From the robust structures of bridges to the intricate circuits of computers, solids underpin our modern world, and a deep understanding of their properties is essential for continued innovation and advancement.
Latest Posts
Latest Posts
-
Classify Each Of The Substances As An Element Or Compound
Apr 01, 2025
-
The Atomic Radii Of The Elements In The Nitrogen Group
Apr 01, 2025
-
What Is The Purpose Of Using A Pedigree
Apr 01, 2025
-
How To Tell If A Function Is Continuous Without Graphing
Apr 01, 2025
-
How To Shift A Function To The Right
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Are The Characteristics Of A Solid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
