Classify Each Of The Substances As An Element Or Compound.
Muz Play
Apr 01, 2025 · 6 min read
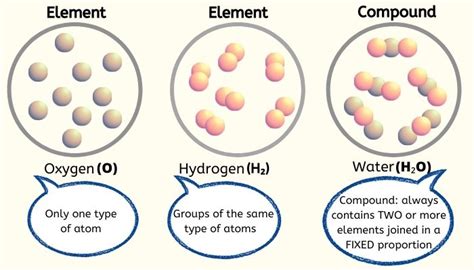
Table of Contents
Classify Each of the Substances as an Element or Compound: A Comprehensive Guide
Understanding the fundamental building blocks of matter is crucial in chemistry. Substances are broadly classified into elements and compounds. This article will delve deep into the distinctions between elements and compounds, providing clear examples and explanations to help you confidently classify various substances. We'll explore the characteristics of each category and examine several common examples to solidify your understanding.
What is an Element?
An element is a pure substance consisting only of atoms that all have the same number of protons in their atomic nuclei. This number, known as the atomic number, uniquely identifies each element. Elements cannot be broken down into simpler substances by chemical means. They are the fundamental building blocks upon which all matter is constructed.
Key Characteristics of Elements:
- Pure substance: Composed of only one type of atom.
- Unique atomic number: Defined by the number of protons in the nucleus.
- Cannot be broken down chemically: Chemical reactions can rearrange elements, but they cannot break them down into smaller, simpler components.
- Represented by symbols: Each element is assigned a unique chemical symbol (e.g., H for hydrogen, O for oxygen, Fe for iron). These symbols are often derived from the element's name, either in English or Latin.
Examples of Elements:
- Hydrogen (H): The lightest and most abundant element in the universe.
- Oxygen (O): Essential for respiration and combustion.
- Carbon (C): Forms the basis of organic chemistry and life as we know it.
- Iron (Fe): A transition metal used in construction and blood.
- Gold (Au): A precious metal valued for its rarity and inertness.
- Chlorine (Cl): A halogen used in water purification and industrial processes.
- Helium (He): A noble gas used in balloons and MRI machines.
- Nitrogen (N): A major component of the Earth's atmosphere.
- Sodium (Na): An alkali metal vital for nerve impulse transmission.
- Uranium (U): A radioactive element used in nuclear energy.
What is a Compound?
A compound is a pure substance composed of two or more different elements chemically bonded together in a fixed ratio. This bonding occurs through the sharing or transfer of electrons between atoms. Unlike mixtures, compounds have a definite and constant composition. Compounds can be broken down into simpler substances (elements) through chemical reactions.
Key Characteristics of Compounds:
- Pure substance: Has a fixed and definite composition.
- Chemical combination of elements: Elements are bonded together through chemical bonds.
- Can be broken down chemically: Chemical reactions can separate the constituent elements.
- Unique properties: Compounds have different properties compared to their constituent elements. For example, water (H₂O) is a liquid at room temperature, while hydrogen and oxygen are gases.
- Represented by chemical formulas: Compounds are represented by chemical formulas that show the types and numbers of atoms present (e.g., H₂O for water, NaCl for table salt).
Examples of Compounds:
- Water (H₂O): Essential for life, composed of hydrogen and oxygen.
- Table salt (NaCl): Sodium chloride, an ionic compound.
- Carbon dioxide (CO₂): A greenhouse gas, composed of carbon and oxygen.
- Glucose (C₆H₁₂O₆): A simple sugar, crucial for energy.
- Ethanol (C₂H₅OH): An alcohol used in beverages and fuels.
- Ammonia (NH₃): Used in fertilizers and cleaning products.
- Methane (CH₄): A major component of natural gas.
- Sulfuric acid (H₂SO₄): A strong acid used in many industrial processes.
- Sodium bicarbonate (NaHCO₃): Baking soda, used in cooking and cleaning.
- Calcium carbonate (CaCO₃): The main component of limestone and marble.
Distinguishing Between Elements and Compounds: A Closer Look
The key difference lies in the nature of the substance: elements are made up of only one type of atom, while compounds are made up of two or more different types of atoms chemically bonded together. This fundamental difference leads to several distinguishing characteristics:
1. Composition:
- Elements: Consist of only one type of atom.
- Compounds: Consist of two or more different types of atoms combined in a fixed ratio.
2. Chemical Breakdown:
- Elements: Cannot be chemically broken down into simpler substances.
- Compounds: Can be chemically broken down into their constituent elements.
3. Properties:
- Elements: Have characteristic properties unique to each element.
- Compounds: Have properties that are different from the properties of their constituent elements.
4. Representation:
- Elements: Represented by chemical symbols (e.g., H, O, Fe).
- Compounds: Represented by chemical formulas (e.g., H₂O, NaCl, CO₂).
Practical Exercises: Classifying Substances
Let's test your understanding with some examples. Classify each of the following substances as an element or a compound:
- Iron (Fe): Element
- Water (H₂O): Compound
- Oxygen (O₂): Element (Note: While O₂ is a diatomic molecule, it's still composed of only one type of atom – oxygen).
- Carbon dioxide (CO₂): Compound
- Gold (Au): Element
- Sodium chloride (NaCl): Compound
- Hydrogen (H₂): Element
- Glucose (C₆H₁₂O₆): Compound
- Helium (He): Element
- Ammonia (NH₃): Compound
- Silver (Ag): Element
- Table sugar (C₁₂H₂₂O₁₁): Compound
- Copper (Cu): Element
- Magnesium (Mg): Element
- Sulfuric acid (H₂SO₄): Compound
- Nitrogen (N₂): Element
- Methane (CH₄): Compound
- Ethanol (C₂H₅OH): Compound
- Aluminum (Al): Element
- Calcium carbonate (CaCO₃): Compound
Beyond the Basics: Isotopes and Allotropes
While the definitions of elements and compounds are relatively straightforward, a deeper understanding requires considering isotopes and allotropes.
Isotopes:
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but a different mass number. For example, carbon-12 (¹²C) and carbon-14 (¹⁴C) are isotopes of carbon. Despite having different numbers of neutrons, isotopes of the same element generally behave the same way chemically. The existence of isotopes doesn't change the classification of a substance as an element.
Allotropes:
Allotropes are different structural forms of the same element. These different forms have different physical and sometimes chemical properties. For example, carbon exists as diamond, graphite, and fullerenes (like buckminsterfullerene). These are all allotropes of carbon – they are all composed solely of carbon atoms, but their arrangement gives them different properties. The existence of allotropes doesn't change the classification of a substance as an element.
Conclusion
Understanding the difference between elements and compounds is fundamental to grasping the basics of chemistry. Elements are the simplest form of matter, while compounds are formed by the chemical combination of two or more elements. By understanding their key characteristics and the examples provided, you can confidently classify substances and build a strong foundation in your understanding of the building blocks of matter. Remember to consider isotopes and allotropes when exploring the complexities of elements. This comprehensive guide should equip you with the knowledge to confidently distinguish between elements and compounds in various contexts.
Latest Posts
Latest Posts
-
Electron Configuration For Copper And Chromium
Apr 02, 2025
-
Confidence Interval Calculator With Two Samples
Apr 02, 2025
-
Lewis Base Vs Bronsted Lowry Base
Apr 02, 2025
-
What Is Max Webers Definition Of Social Status Based On
Apr 02, 2025
-
How To Calculate External Financing Needed
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Classify Each Of The Substances As An Element Or Compound. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
