What Does A Negative Enthalpy Mean
Muz Play
Mar 27, 2025 · 6 min read
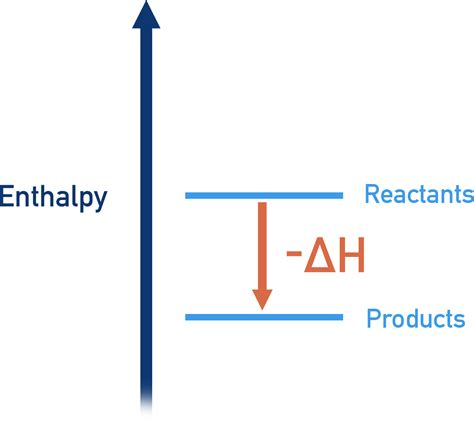
Table of Contents
What Does a Negative Enthalpy Mean? Understanding Exothermic Reactions and Energy Changes
Enthalpy, denoted by the symbol 'H', is a fundamental thermodynamic property that plays a crucial role in understanding energy changes during chemical and physical processes. While the concept itself might seem abstract, understanding what a negative enthalpy signifies is key to comprehending the behavior of numerous reactions and phenomena in our world. This article will delve into the meaning of negative enthalpy, explaining its implications in exothermic reactions, providing real-world examples, and discussing its importance in various scientific fields.
Understanding Enthalpy: A Quick Recap
Before diving into the specifics of negative enthalpy, let's briefly revisit the definition of enthalpy. Enthalpy is essentially the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the current state of the system, not on the path taken to reach that state. The change in enthalpy (ΔH) during a process represents the heat exchanged between the system and its surroundings at constant pressure.
This exchange of heat is crucial. A system can either release heat to its surroundings or absorb heat from them. These exchanges directly relate to the sign of ΔH:
- Positive ΔH (Endothermic): The system absorbs heat from its surroundings. The reaction feels cold.
- Negative ΔH (Exothermic): The system releases heat to its surroundings. The reaction feels hot.
Decoding Negative Enthalpy: The Exothermic Nature
A negative enthalpy change (ΔH < 0) signifies an exothermic process. In an exothermic reaction, the enthalpy of the products is lower than the enthalpy of the reactants. This means that energy is released during the reaction, often in the form of heat. Think of it like this: the system is losing energy, and that energy is transferred to the surroundings, increasing the surroundings' temperature.
This release of energy is what characterizes exothermic reactions. The magnitude of the negative enthalpy value indicates the amount of heat released. A larger negative value means more heat is released, making the reaction more exothermic.
Visualizing Exothermic Reactions: Energy Diagrams
Energy diagrams provide a helpful visual representation of exothermic reactions. They show the energy levels of the reactants and products, with the difference representing the enthalpy change. In an exothermic reaction, the energy level of the products is lower than that of the reactants, represented by a downward slope on the diagram. The difference in energy between the reactants and products is the magnitude of the negative enthalpy change.
[Insert an image here showing a typical energy diagram for an exothermic reaction. The diagram should clearly illustrate the reactants at a higher energy level than the products, with the energy difference labeled as -ΔH.]
Real-World Examples of Negative Enthalpy
Exothermic reactions are prevalent in our everyday lives and in numerous industrial processes. Here are some notable examples:
1. Combustion Reactions:
Combustion, the rapid reaction of a substance with oxygen, is a highly exothermic process. The burning of fuels like wood, propane, or gasoline releases significant amounts of heat, making them useful for heating and powering engines. The negative enthalpy change in combustion is substantial, resulting in a noticeable temperature increase.
2. Neutralization Reactions:
When a strong acid reacts with a strong base, a neutralization reaction occurs. This reaction releases a considerable amount of heat, resulting in a negative enthalpy change. This is why mixing strong acids and bases can be dangerous—the heat generated can be substantial enough to cause burns.
3. Formation of Chemical Bonds:
The formation of chemical bonds is generally an exothermic process. When atoms bond together, they release energy as they become more stable. This energy release manifests as a negative enthalpy change. The stronger the bond formed, the more negative the enthalpy change will be.
4. Respiration:
Even biological processes are characterized by enthalpy changes. Cellular respiration, the process by which living organisms convert glucose into energy, is a complex series of exothermic reactions. The release of energy during respiration fuels our bodily functions.
5. Condensation:
The phase transition from gas to liquid (condensation) is another example of an exothermic process. When water vapor condenses into liquid water, it releases heat to the surroundings. This is why condensation can feel warm on a cold surface.
Beyond Heat: Other Manifestations of Negative Enthalpy
While heat is the most common manifestation of an exothermic reaction (and therefore a negative enthalpy change), energy can be released in other forms too. For instance:
- Light: Some exothermic reactions release energy as light, as seen in chemiluminescence (e.g., glow sticks).
- Sound: Certain explosive reactions release energy as sound, alongside heat.
- Electrical Energy: Some electrochemical reactions release electrical energy, such as in batteries.
These examples highlight that a negative enthalpy change doesn't solely indicate heat release; it signifies a net release of energy in any form.
Applications of Negative Enthalpy in Various Fields
Understanding negative enthalpy is crucial across various scientific and technological domains:
1. Chemistry:
In chemistry, understanding enthalpy changes is vital for predicting reaction spontaneity, designing efficient chemical processes, and determining reaction equilibrium. Exothermic reactions are often favored in industrial settings because of their energy release.
2. Engineering:
Engineers utilize enthalpy data in designing engines, power plants, and other systems that rely on heat generation from exothermic reactions. They help determine the efficiency and safety of such systems.
3. Materials Science:
Enthalpy plays a key role in understanding the properties of materials. The enthalpy changes during phase transitions, such as melting and solidification, can be used to design materials with specific characteristics.
4. Environmental Science:
Understanding exothermic reactions is crucial for evaluating the environmental impact of various processes. For example, assessing the heat released during combustion helps determine the effects of fossil fuel usage on climate change.
Factors Affecting Enthalpy Changes
Several factors can influence the enthalpy change of a reaction:
- Temperature: The enthalpy change can vary with temperature.
- Pressure: Especially for reactions involving gases, pressure can significantly affect enthalpy change.
- State of reactants and products: The physical state (solid, liquid, gas) of reactants and products directly influences enthalpy changes.
Conclusion: The Significance of Negative Enthalpy
A negative enthalpy change signifies an exothermic reaction, where the system releases energy to its surroundings. This release can manifest as heat, light, sound, or electrical energy. Understanding the meaning and implications of negative enthalpy is crucial across numerous scientific and technological fields, impacting areas from energy production to materials science to environmental studies. By grasping the concept of exothermic reactions and the underlying energy changes, we gain a deeper understanding of the world around us and the processes that shape it. The ability to predict and control exothermic reactions is vital for technological advancement and ensuring safety in various applications. Further exploration of enthalpy and its associated concepts is essential for continued progress in numerous scientific disciplines.
Latest Posts
Latest Posts
-
What Is The Difference Between Empirical Formula And Molecular Formula
Mar 30, 2025
-
What Is The Function Of A State
Mar 30, 2025
-
How Are Vestigial Structures An Example Of Evidence Of Evolution
Mar 30, 2025
-
What Is The Communication Accommodation Theory
Mar 30, 2025
-
How Many Molecules Are In A Drop Of Water
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Does A Negative Enthalpy Mean . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
