How Many Molecules Are In A Drop Of Water
Muz Play
Mar 30, 2025 · 5 min read
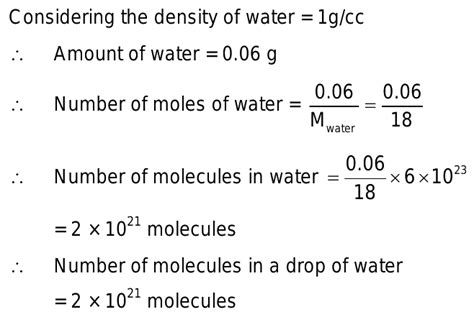
Table of Contents
How Many Molecules Are in a Drop of Water? A Deep Dive into Avogadro's Number and Water's Molecular Structure
Have you ever wondered about the sheer number of tiny particles that make up something as seemingly simple as a single drop of water? It's a question that delves into the fascinating world of chemistry, physics, and the incredible scale of the universe at the molecular level. This article will explore exactly how many molecules are in a drop of water, explaining the concepts and calculations involved along the way. We'll also touch upon the implications of this vast number and its relevance in various scientific fields.
Understanding Avogadro's Number: The Key to Counting Molecules
The answer to our question hinges on understanding Avogadro's number, a fundamental constant in chemistry. Avogadro's number (approximately 6.022 x 10<sup>23</sup>) represents the number of constituent particles (atoms, molecules, ions, etc.) in one mole of a substance. A mole is a unit of measurement in chemistry that defines an amount of substance containing the same number of entities as there are atoms in 12 grams of carbon-12. In simpler terms, it's a convenient way to handle extremely large numbers of incredibly small particles.
Think of it like this: if you had a dozen eggs, you have 12 eggs. If you have a mole of eggs (hypothetically, of course!), you'd have 6.022 x 10<sup>23</sup> eggs – an unimaginably vast quantity. Similarly, a mole of water contains Avogadro's number of water molecules.
The Molecular Structure of Water: H₂O
Before we can calculate the number of molecules, we need to understand the composition of a water molecule. Water, denoted by the chemical formula H₂O, consists of two hydrogen atoms covalently bonded to one oxygen atom. This simple yet crucial molecule is the building block of all the water we encounter in our daily lives. The specific arrangement of these atoms and their interactions create unique properties of water, such as its high surface tension, excellent solvent capabilities, and anomalous density behavior. Understanding the H₂O structure is crucial for our calculations.
Determining the Mass of a Drop of Water: The Starting Point
To calculate the number of molecules in a drop of water, we first need to determine the mass of a typical drop. The size of a drop can vary depending on various factors such as the method of dispensing, temperature, and the liquid's properties. For our calculations, let's assume an average drop of water weighs approximately 0.05 grams (50 milligrams). This is a reasonable approximation for many everyday scenarios.
From Grams to Moles: Using Molar Mass
Next, we need to convert the mass of the water drop from grams to moles. This involves using the molar mass of water. The molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). For water (H₂O), the molar mass is approximately 18.015 g/mol. This is calculated by adding the atomic masses of two hydrogen atoms (1.008 g/mol each) and one oxygen atom (15.999 g/mol).
To find the number of moles in our 0.05-gram water drop, we use the following formula:
Moles = Mass (grams) / Molar Mass (g/mol)
Moles = 0.05 g / 18.015 g/mol ≈ 0.002775 moles
From Moles to Molecules: Applying Avogadro's Number
Now that we know the number of moles in our water drop, we can finally calculate the number of molecules using Avogadro's number:
Number of Molecules = Moles × Avogadro's Number
Number of Molecules ≈ 0.002775 moles × 6.022 x 10<sup>23</sup> molecules/mole ≈ 1.67 x 10<sup>21</sup> molecules
Therefore, there are approximately 1.67 x 10<sup>21</sup> molecules in a 0.05-gram drop of water. This is an incredibly large number, highlighting the microscopic scale of molecules and the immense number of them present even in a tiny amount of a substance.
Variations and Considerations: The Importance of Precision
It's crucial to understand that the number we calculated is an approximation. The mass of a water drop can vary significantly, leading to variations in the final result. Furthermore, the assumption of a perfectly pure water sample is rarely met in reality. Impurities and dissolved substances can affect the overall mass and, consequently, the number of water molecules. The temperature also influences the density of water, affecting the mass of a given volume.
More precise calculations would require careful measurement of the water drop's mass using highly accurate instruments and considering the temperature and purity of the water sample. For scientific applications where precise measurements are crucial, these factors must be taken into account.
The Implications of Avogadro's Number and Molecular Scale
The ability to calculate the number of molecules in a drop of water underscores the power of Avogadro's number and its importance in chemistry and related fields. This principle allows scientists to bridge the gap between macroscopic quantities (grams, liters) and microscopic entities (atoms, molecules). It is essential for various applications, including:
- Stoichiometry: Calculating the amounts of reactants and products in chemical reactions.
- Solution Chemistry: Determining the concentrations of substances in solutions.
- Thermodynamics: Understanding energy changes at the molecular level.
- Material Science: Analyzing the properties of materials based on their molecular structure.
Beyond Water: Applying the Concept to Other Substances
The principles outlined above can be applied to calculate the number of molecules in any substance, provided you know its molar mass and the mass of the sample. Simply follow the same steps: convert the mass to moles using the molar mass and then multiply by Avogadro's number to obtain the number of molecules.
Conclusion: A Microscopic World Revealed
The seemingly simple question of "how many molecules are in a drop of water?" opens a door to a vast and fascinating world of molecular interactions and chemical principles. Understanding Avogadro's number and the methods for calculating the number of molecules in a sample is fundamental to grasping the essence of chemistry and its applications in various scientific disciplines. While the exact number may vary slightly based on experimental conditions, the sheer magnitude of the number underscores the immense scale of the microscopic world and the power of quantitative analysis in exploring it. The concept extends far beyond water, offering a powerful tool for understanding the composition and properties of all matter.
Latest Posts
Latest Posts
-
Classify Each Of The Substances As An Element Or Compound
Apr 01, 2025
-
The Atomic Radii Of The Elements In The Nitrogen Group
Apr 01, 2025
-
What Is The Purpose Of Using A Pedigree
Apr 01, 2025
-
How To Tell If A Function Is Continuous Without Graphing
Apr 01, 2025
-
How To Shift A Function To The Right
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Molecules Are In A Drop Of Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
