How Many Times Smaller Is An Electron Than A Proton
Muz Play
Apr 01, 2025 · 6 min read
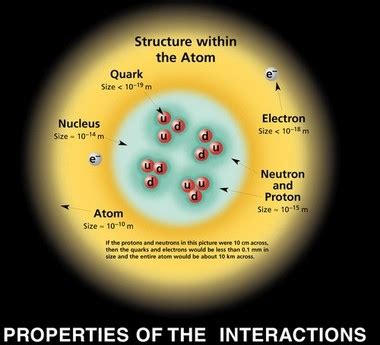
Table of Contents
How Many Times Smaller is an Electron Than a Proton? Delving into the Subatomic World
The question of how much smaller an electron is than a proton is a fundamental one in understanding the structure of matter. While seemingly simple, the answer requires exploring the fascinating world of subatomic particles, their properties, and the units used to measure them. This journey will delve deep into the realm of quantum mechanics and reveal the intricacies of these fundamental building blocks of the universe.
Understanding Subatomic Particles: Electrons and Protons
Before we delve into the size comparison, let's first establish a clear understanding of electrons and protons. Both are fundamental particles, meaning they are not composed of smaller constituents (as far as we currently know). They are key components of atoms, the basic units of all matter.
The Electron: A Tiny, Negatively Charged Particle
The electron carries a single unit of negative electric charge. Its discovery revolutionized our understanding of matter, proving that atoms were not indivisible as previously thought. Electrons are remarkably lightweight and are involved in chemical bonding and electrical conductivity. Their movement generates electric currents, powering everything from our smartphones to power grids.
The Proton: A Positively Charged, Relatively Massive Particle
Protons reside within the atom's nucleus, along with neutrons. They carry a single unit of positive electric charge, exactly balancing the negative charge of electrons in a neutral atom. Protons are significantly more massive than electrons, contributing the majority of an atom's mass. They are crucial for determining an atom's identity (its atomic number).
Comparing Sizes: The Challenge of Defining "Size" at the Subatomic Level
The difficulty in comparing the sizes of electrons and protons lies in the very nature of these particles. Unlike macroscopic objects with clearly defined boundaries, subatomic particles exhibit wave-particle duality. This means they behave both as particles and as waves, making the concept of "size" less straightforward.
Instead of a classical radius, physicists often use a concept called the "charge radius." This represents the effective size of the particle's electric charge distribution. It's important to note that this is not a physical size in the traditional sense but a measure of the spatial extent of the particle's influence.
The Mass Difference: A Dramatic Disparity
While the size comparison is complex, the mass difference between electrons and protons is straightforward. The proton is approximately 1836 times more massive than the electron. This massive difference in mass has significant implications for their behavior and interactions within an atom.
Exploring the Charge Radius: A More Nuanced Approach to Size
The charge radius of a proton is approximately 0.877 femtometers (fm), where 1 fm = 10⁻¹⁵ meters. Determining the charge radius of an electron is far more complex. Due to its wave-like nature and lack of a clearly defined boundary, its size is better understood as a probability distribution rather than a solid boundary.
While an electron doesn't have a definitively measured radius in the same way a proton does, we can use various theoretical approaches and experimental observations to make comparisons. These methods suggest that the electron's charge radius is far smaller than the proton's, potentially even vanishingly small, approaching zero. The exact value remains a subject of ongoing research and debate among physicists.
The Role of Quantum Mechanics: Understanding Uncertainty
The challenge in defining the size of subatomic particles stems from the principles of quantum mechanics. The Heisenberg uncertainty principle states that we cannot simultaneously know both the position and momentum of a particle with perfect accuracy. The more precisely we know one, the less precisely we know the other. This inherent uncertainty makes it difficult to pinpoint the exact boundaries of a particle like an electron.
Experimental Methods: Peering into the Subatomic Realm
Scientists use sophisticated experimental techniques to indirectly probe the sizes and properties of subatomic particles. These techniques include:
- Electron scattering experiments: By scattering high-energy electrons off protons (and vice-versa), physicists can infer information about their internal structure and charge distribution.
- Spectroscopy: Analyzing the light emitted or absorbed by atoms and molecules provides clues about the energy levels of electrons and the interactions within atoms.
- Muon g-2 experiment: Precision measurements of the magnetic moment of the muon, a heavier cousin of the electron, help refine our understanding of fundamental particle properties and interactions.
Beyond Size: Other Key Differences Between Electrons and Protons
While size is a crucial aspect, several other critical differences exist between electrons and protons:
- Charge: Electrons carry a negative charge, while protons carry a positive charge.
- Mass: Protons are significantly more massive than electrons.
- Location within the atom: Electrons reside in orbitals surrounding the nucleus, while protons are found within the nucleus.
- Fundamental nature: Both are considered fundamental particles, meaning they are not composed of smaller constituents (as far as we currently know).
- Interactions: Electrons participate primarily in electromagnetic interactions, while protons participate in both electromagnetic and strong nuclear interactions (the force that binds protons and neutrons together in the nucleus).
The Ongoing Quest for Precision: Unanswered Questions and Future Research
Despite decades of research, some questions about the exact size and properties of electrons and protons remain. Ongoing research focuses on:
- Precisely measuring the electron's charge radius: Refining our understanding of this elusive quantity will help deepen our understanding of fundamental physics.
- Exploring the proton's internal structure: Scientists are still working to fully understand the complex internal dynamics of protons, including the role of quarks and gluons.
- The role of Quantum Chromodynamics (QCD): A comprehensive theoretical framework is required to accurately describe the behavior of quarks and gluons that make up protons.
Conclusion: A Journey into the Heart of Matter
In conclusion, while a definitive answer to "how many times smaller is an electron than a proton?" is challenging due to the complexities of quantum mechanics and the definition of "size" at the subatomic level, we can confidently state that the electron is considerably smaller than the proton, and its charge radius is many orders of magnitude less than that of a proton. The proton's charge radius is approximately 0.877 fm, whereas the electron's effective radius is considered far smaller, approaching zero. Furthermore, a proton is approximately 1836 times more massive than an electron. The ongoing research in this field continues to refine our understanding of these fundamental particles and their roles in shaping the universe we inhabit. The quest to unravel the mysteries of the subatomic world is a testament to human curiosity and our relentless pursuit of knowledge. The complexities and challenges involved only serve to emphasize the wonder and beauty of the quantum realm.
Latest Posts
Latest Posts
-
How Is Chemistry Used In Forensic Science
Apr 02, 2025
-
How Do You Square A Radical
Apr 02, 2025
-
Was Adolf Hitler A Good Leader
Apr 02, 2025
-
Is Cholesterol A Carbohydrate Protein Or Lipid
Apr 02, 2025
-
Pick A Number Between 1 And 51
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Times Smaller Is An Electron Than A Proton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
