How Many Unhybridized P Orbitals In Sp
Muz Play
Mar 16, 2025 · 6 min read
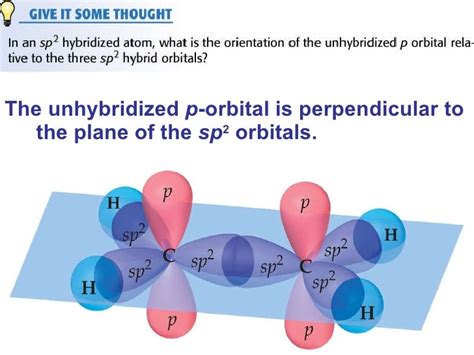
Table of Contents
How Many Unhybridized p Orbitals in sp Hybridization?
Understanding hybridization is crucial for grasping the structure and bonding in molecules. This article delves deep into the concept of sp hybridization, specifically addressing the number of unhybridized p orbitals remaining after the hybridization process. We'll explore the mechanics of sp hybridization, its implications for molecular geometry, and the role of unhybridized p orbitals in forming pi (π) bonds.
Understanding Hybridization: A Foundation
Hybridization is a theoretical concept in chemistry that explains the bonding in molecules more accurately than simple valence bond theory alone. It involves the mixing of atomic orbitals within an atom to create new hybrid orbitals that are energetically more favorable for bonding. The number and type of hybrid orbitals formed depend on the number and types of atomic orbitals involved in the mixing.
The most common types of hybridization include:
- sp<sup>3</sup> hybridization: One s orbital and three p orbitals combine to form four sp<sup>3</sup> hybrid orbitals. This is common in molecules like methane (CH<sub>4</sub>).
- sp<sup>2</sup> hybridization: One s orbital and two p orbitals combine to form three sp<sup>2</sup> hybrid orbitals. One p orbital remains unhybridized. This is found in molecules like ethene (C<sub>2</sub>H<sub>4</sub>).
- sp hybridization: One s orbital and one p orbital combine to form two sp hybrid orbitals. Two p orbitals remain unhybridized. This is characteristic of molecules like ethyne (C<sub>2</sub>H<sub>2</sub>).
Delving into sp Hybridization
In sp hybridization, one s orbital and one p orbital from the valence shell of an atom combine to generate two equivalent sp hybrid orbitals. These hybrid orbitals are linear and oriented 180 degrees apart. This arrangement is crucial for forming strong sigma (σ) bonds.
The Key Players: s and p Orbitals
Before hybridization, the atom possesses one s orbital and three p orbitals (p<sub>x</sub>, p<sub>y</sub>, p<sub>z</sub>) in its valence shell. These orbitals have different shapes and energies. The s orbital is spherical, while the p orbitals are dumbbell-shaped and oriented along the x, y, and z axes.
The Hybridization Process
During sp hybridization, the s orbital and one of the p orbitals (let's say p<sub>x</sub>) mix to form two new sp hybrid orbitals. These hybrid orbitals are now a blend of s and p orbital characteristics; they possess some s character and some p character. The remaining two p orbitals (p<sub>y</sub> and p<sub>z</sub>) remain unchanged and are referred to as unhybridized p orbitals.
The Significance of Unhybridized p Orbitals in sp Hybridization
The crucial point here is that two unhybridized p orbitals remain after sp hybridization. These unhybridized p orbitals play a vital role in the formation of pi (π) bonds.
Pi (π) Bond Formation
While sp hybrid orbitals are used to form strong sigma (σ) bonds, the unhybridized p orbitals participate in the formation of weaker pi (π) bonds. These π bonds are formed by the sideways overlap of the unhybridized p orbitals from adjacent atoms. This sideways overlap is less effective than the end-to-end overlap of hybrid orbitals in sigma bonds, leading to weaker bonds.
Multiple Bonds and sp Hybridization
The presence of two unhybridized p orbitals on each carbon atom in sp-hybridized molecules allows for the formation of two pi bonds with another atom. This explains why molecules exhibiting sp hybridization often have triple bonds (e.g., ethyne, C<sub>2</sub>H<sub>2</sub>). Each carbon atom contributes one sp hybrid orbital to form a sigma bond with the other carbon atom and a sigma bond to a hydrogen atom. The remaining two unhybridized p orbitals from each carbon atom then overlap laterally, forming two pi bonds. Therefore, the carbon-carbon triple bond comprises one sigma bond and two pi bonds.
Examples in Organic Chemistry
Sp hybridization is prevalent in many organic molecules with triple bonds or cumulative double bonds. Examples include:
- Ethyne (acetylene, C<sub>2</sub>H<sub>2</sub>): Each carbon atom is sp hybridized, forming one sigma bond with the other carbon atom and one sigma bond with a hydrogen atom. The two remaining unhybridized p orbitals on each carbon form two pi bonds between the carbon atoms.
- Cyanide ion (CN<sup>-</sup>): The carbon atom is sp hybridized, forming a sigma bond with the nitrogen atom. The two unhybridized p orbitals form two pi bonds between the carbon and nitrogen atoms. Note that the nitrogen atom is also involved in multiple bonding here.
- Allenes: Molecules containing cumulene structures (like allene, CH<sub>2</sub>=C=CH<sub>2</sub>) show sp hybridization in the central carbon atom. The terminal carbons are sp<sup>2</sup> hybridized.
Visualizing sp Hybridization and Unhybridized p Orbitals
To fully grasp the concept, visualizing the orbitals is essential. Imagine the s orbital as a sphere and the p orbitals as dumbbells oriented along the x, y, and z axes. During sp hybridization, the s orbital and one p orbital combine to form two linear sp hybrid orbitals, leaving two unhybridized p orbitals available for pi bond formation. These can be represented through various molecular modelling software or drawn using standard chemical notation.
Addressing Common Misconceptions
A frequent misunderstanding is that the unhybridized p orbitals somehow “disappear” or are not part of the bonding picture. This is incorrect. While they don't participate directly in sigma bond formation, they are crucial for pi bond formation, leading to multiple bonds. The hybridization model simply simplifies visualizing the electron distribution and bonding arrangement.
Another misconception is confusing the number of unhybridized orbitals with the number of bonds formed. The number of unhybridized p orbitals dictates the potential for pi bond formation, not necessarily the number of bonds formed. The actual number of bonds is dependent on other factors, such as the availability of electrons and the presence of other atoms capable of forming pi bonds.
Beyond Organic Chemistry: Applications in Inorganic Chemistry
While the examples above focus on organic molecules, sp hybridization isn't restricted to organic chemistry. Many inorganic compounds also exhibit sp hybridization, demonstrating the broader applicability of this concept in understanding chemical bonding. Examples include certain metal complexes and linear diatomic molecules.
Conclusion: The Crucial Role of Unhybridized p Orbitals in sp Hybridization
In conclusion, the sp hybridization model explains that two unhybridized p orbitals remain after the hybridization process. These unhybridized orbitals are instrumental in forming pi bonds, contributing significantly to the stability and properties of molecules containing multiple bonds. Understanding the presence and role of these unhybridized p orbitals is essential for correctly predicting and interpreting the molecular geometry, bonding, and reactivity of numerous compounds in both organic and inorganic chemistry. Remembering that two unhybridized p orbitals are key to the existence of triple bonds and the unique characteristics of sp hybridized molecules provides a solid foundation for further exploration of advanced chemistry concepts.
Latest Posts
Latest Posts
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Unhybridized P Orbitals In Sp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
