How Many Valence Electrons In B
Muz Play
Mar 30, 2025 · 6 min read
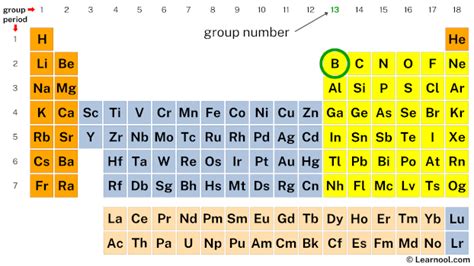
Table of Contents
How Many Valence Electrons Does Boron (B) Have? A Deep Dive into Atomic Structure and Bonding
Boron, a metalloid element with the symbol 'B' and atomic number 5, plays a crucial role in various scientific fields. Understanding its electronic structure, particularly the number of valence electrons, is key to comprehending its chemical behavior and the properties of its compounds. This comprehensive article will delve deep into the world of boron's valence electrons, explaining the concept, its significance, and how it influences boron's reactivity and bonding characteristics.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before we focus specifically on boron, let's establish a firm grasp of the concept of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the ones most involved in chemical bonding and determine an element's reactivity. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, often following the octet rule (eight electrons in the outermost shell), although there are exceptions, particularly with elements that have fewer than eight valence electrons.
The number of valence electrons directly relates to an element's position on the periodic table. For the main group elements (Groups 1-18, excluding transition metals), the group number (using the American system of numbering) usually corresponds to the number of valence electrons. However, this isn't a hard and fast rule for all elements and especially isn't a rule for the transition metals.
Determining Boron's Valence Electrons: Electronic Configuration and the Periodic Table
Boron, situated in Group 13 (or IIIA) of the periodic table, provides a straightforward example. Its atomic number of 5 indicates that it has five protons and five electrons in a neutral atom. To determine the number of valence electrons, we need to examine its electronic configuration.
The electronic configuration of boron is 1s²2s²2p¹. This notation tells us the distribution of electrons across different energy levels and sublevels within the atom.
- 1s²: Two electrons occupy the first energy level (n=1) in the 's' subshell.
- 2s²: Two electrons occupy the second energy level (n=2) in the 's' subshell.
- 2p¹: One electron occupies the second energy level (n=2) in the 'p' subshell.
The outermost shell for boron is the second energy level (n=2), which contains a total of three electrons (two from 2s and one from 2p). Therefore, boron has three valence electrons.
Visualizing Boron's Electronic Structure
Imagine the atom as a nucleus surrounded by electron shells. The first shell (n=1) can hold a maximum of two electrons, while the second shell (n=2) can accommodate up to eight. Boron, with its five electrons, fills the first shell completely and partially fills the second shell with three valence electrons. This incomplete outermost shell explains boron's tendency to participate in chemical reactions.
Boron's Chemical Behavior: The Influence of Valence Electrons
The three valence electrons significantly influence boron's chemical behavior and bonding preferences. Boron readily forms covalent bonds, sharing its three valence electrons with other atoms to achieve a more stable electron configuration. It rarely loses all three electrons to form a +3 ion because removing those electrons requires a significant amount of energy. While B<sup>3+</sup> exists in some compounds, it is not a common form of the element. The covalent bonding preference leads to various interesting properties and compounds.
Covalent Bonding in Boron Compounds: Examples
Boron's covalent bonding is evident in a wide range of compounds, including:
-
Boron trifluoride (BF₃): Boron shares its three valence electrons with three fluorine atoms, forming three covalent bonds. Each fluorine atom contributes one electron to the bond, resulting in a stable octet for each fluorine atom. Note that this is a notable exception to the octet rule as Boron only achieves six electrons in its valence shell. This is due to the high electronegativity of the fluorine atom.
-
Boron trichloride (BCl₃): Similar to BF₃, boron shares its three valence electrons with three chlorine atoms, forming three covalent bonds.
-
Boranes: These compounds are composed of boron and hydrogen atoms, exhibiting various bonding structures, including electron-deficient bonds where boron atoms share fewer than eight electrons. The study of boranes has significantly contributed to the understanding of chemical bonding. Examples of this include diborane (B₂H₆).
-
Boron oxides: Boron forms various oxides, such as B₂O₃, where boron atoms share their valence electrons with oxygen atoms.
The Role of Boron in Other Chemical Reactions
Boron's three valence electrons also make it a versatile element participating in other chemical reactions:
-
Formation of borates: Boron readily reacts with various metals and nonmetals, forming borates which are salts of boric acid (H₃BO₃). These borates have various applications in detergents and glasses.
-
Doping in Semiconductors: Boron is frequently used as a p-type dopant in semiconductors like silicon. By replacing some silicon atoms in the crystal lattice, boron introduces "holes" (positive charge carriers), enhancing the semiconductor's electrical conductivity. This is a key aspect of semiconductor device fabrication.
-
Other applications: Boron compounds are also used in various other applications, including fertilizers, insecticides, and nuclear reactors.
Boron's Unique Bonding Capabilities: Beyond the Octet Rule
While the octet rule serves as a useful guideline, boron, with its three valence electrons, often forms compounds that deviate from this rule. This is because it's energetically favorable for boron to engage in electron deficient bonding. This often results in interesting structural features. Many boron compounds involve three-center two-electron (3c-2e) bonds. In these bonds, two electrons are shared among three atoms, leading to electron deficiency around the boron atoms. The classic example is diborane (B₂H₆).
The ability of boron to form electron-deficient bonds and deviate from the octet rule is crucial for its diverse chemistry and its role in many important compounds and materials. Understanding these aspects is paramount for anyone working with boron or boron-containing materials.
Conclusion: The Significance of Boron's Three Valence Electrons
In summary, boron possesses three valence electrons, a fact that fundamentally shapes its chemical behavior. Its tendency to form covalent bonds, often leading to electron-deficient structures, underlies the formation of a wide array of compounds with significant applications in diverse fields. From the creation of semiconductors to the production of borates, boron's three valence electrons play a central role. This exploration of boron's electronic structure serves as a foundational understanding of its chemistry and the multitude of its applications. Further research into boron's unique chemical properties continues to reveal new applications and further our understanding of this fascinating metalloid element. The simple number three, representing its valence electrons, unlocks a world of complex and fascinating chemical behavior.
Latest Posts
Latest Posts
-
What Is The Definition Of Form In Music
Apr 01, 2025
-
Staph Epidermidis Hemolysis On Blood Agar
Apr 01, 2025
-
Difference Between Autonomic And Somatic Nervous System
Apr 01, 2025
-
Phase Change Properties Of Pure Substances
Apr 01, 2025
-
Where Do Antimicrobial Drugs Come From
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In B . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
