Phase Change Properties Of Pure Substances
Muz Play
Apr 01, 2025 · 6 min read
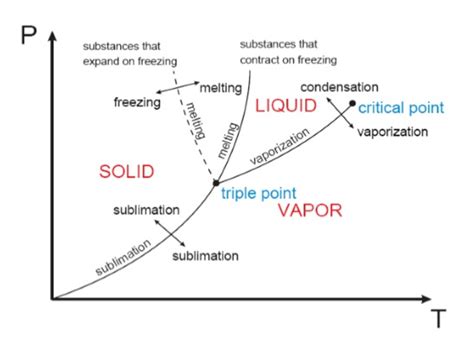
Table of Contents
Phase Change Properties of Pure Substances: A Deep Dive
Understanding the phase change properties of pure substances is fundamental to numerous engineering disciplines, from chemical processing and power generation to materials science and refrigeration. This comprehensive guide delves into the intricate details of these properties, exploring the underlying principles and their practical applications. We will examine phase diagrams, thermodynamic relationships, and the impact of pressure and temperature on phase transitions.
What are Phase Changes?
A phase change, or phase transition, refers to the transformation of a substance from one physical state (phase) to another. The most common phases are solid, liquid, and gas, although others exist, such as plasma and Bose-Einstein condensates. These transitions are driven by changes in temperature, pressure, or both, and involve a significant alteration in the substance's molecular arrangement and energy content.
Common Phase Transitions:
- Melting/Freezing: The transition between the solid and liquid phases. Melting involves absorbing heat (endothermic), while freezing releases heat (exothermic).
- Vaporization/Condensation: The transition between the liquid and gas phases. Vaporization (boiling or evaporation) requires energy input, while condensation releases energy.
- Sublimation/Deposition: The direct transition between the solid and gas phases, bypassing the liquid phase. Sublimation requires energy, while deposition releases it.
Phase Diagrams: Visualizing Phase Transitions
Phase diagrams are graphical representations that illustrate the conditions (temperature and pressure) under which a substance exists in different phases. They are invaluable tools for understanding phase transitions and predicting the behavior of substances under varying conditions. A typical phase diagram for a pure substance shows regions corresponding to the solid, liquid, and gaseous phases, separated by curves representing phase boundaries.
Key Features of a Phase Diagram:
- Solid-Liquid Equilibrium Curve (Melting/Freezing Curve): This curve represents the conditions under which the solid and liquid phases coexist in equilibrium. The melting point increases with increasing pressure for most substances, but water is a notable exception.
- Liquid-Vapor Equilibrium Curve (Boiling/Condensation Curve): This curve shows the conditions where the liquid and vapor phases are in equilibrium. The boiling point increases with increasing pressure.
- Solid-Vapor Equilibrium Curve (Sublimation/Deposition Curve): This curve depicts the conditions where the solid and vapor phases are in equilibrium.
- Triple Point: The unique point where all three phases (solid, liquid, and vapor) coexist in equilibrium.
- Critical Point: The point beyond which the distinction between liquid and gas phases disappears. Above the critical temperature and pressure, the substance exists as a supercritical fluid.
Thermodynamic Properties and Phase Changes
Phase transitions are governed by thermodynamic principles, specifically enthalpy and entropy changes.
Enthalpy of Phase Change:
The enthalpy of phase change (ΔH) represents the heat absorbed or released during a phase transition at constant pressure. It is expressed in units of Joules per mole (J/mol) or kilojoules per mole (kJ/mol). Specific enthalpy changes are denoted as follows:
- Enthalpy of Fusion (ΔH<sub>fus</sub>): Heat absorbed during melting.
- Enthalpy of Vaporization (ΔH<sub>vap</sub>): Heat absorbed during vaporization.
- Enthalpy of Sublimation (ΔH<sub>sub</sub>): Heat absorbed during sublimation.
The relationship between these enthalpy changes is given by: ΔH<sub>sub</sub> = ΔH<sub>fus</sub> + ΔH<sub>vap</sub>.
Entropy of Phase Change:
The entropy of phase change (ΔS) reflects the change in disorder or randomness during the transition. It is expressed in units of Joules per mole-Kelvin (J/mol·K). An increase in entropy signifies an increase in disorder, which is characteristic of phase transitions from solid to liquid and liquid to gas.
The relationship between enthalpy and entropy changes during a phase transition at equilibrium is given by: ΔS = ΔH/T, where T is the temperature in Kelvin.
Clausius-Clapeyron Equation: Modeling Phase Equilibrium
The Clausius-Clapeyron equation provides a mathematical relationship between the vapor pressure of a substance and its temperature along the liquid-vapor equilibrium curve. This equation is crucial for predicting boiling points at different pressures and understanding the behavior of substances in various environments.
The equation is typically expressed as:
dP/dT = ΔH<sub>vap</sub> / (TΔV<sub>vap</sub>)
Where:
- dP/dT is the slope of the vapor pressure curve.
- ΔH<sub>vap</sub> is the enthalpy of vaporization.
- T is the temperature in Kelvin.
- ΔV<sub>vap</sub> is the change in molar volume between the liquid and vapor phases.
A simplified form of the Clausius-Clapeyron equation, often used for approximations, is:
ln(P<sub>2</sub>/P<sub>1</sub>) = -ΔH<sub>vap</sub>/R * (1/T<sub>2</sub> - 1/T<sub>1</sub>)
Where:
- P<sub>1</sub> and P<sub>2</sub> are the vapor pressures at temperatures T<sub>1</sub> and T<sub>2</sub>, respectively.
- R is the ideal gas constant.
Impact of Pressure and Temperature
Pressure and temperature play pivotal roles in determining the phase of a substance.
Effect of Pressure:
Increasing pressure generally favors the denser phase. Therefore, increasing pressure favors the solid phase over the liquid phase and the liquid phase over the gaseous phase. However, water is an exception; increasing pressure at temperatures below 4°C causes ice to melt.
Effect of Temperature:
Increasing temperature generally increases the kinetic energy of molecules, leading to phase transitions from solid to liquid and liquid to gas. The specific temperatures at which these transitions occur (melting point and boiling point) are dependent on the pressure.
Practical Applications of Phase Change Properties
The phase change properties of pure substances are exploited in a vast array of applications:
- Refrigeration and Air Conditioning: These systems utilize the enthalpy of vaporization of refrigerants to absorb heat and cool spaces.
- Power Generation: Steam power plants rely on the enthalpy of vaporization of water to generate electricity.
- Material Processing: Phase transitions are crucial in various material processing techniques, such as casting, welding, and crystal growth.
- Chemical Engineering: Understanding phase equilibria is vital for designing and optimizing chemical processes, including distillation, extraction, and crystallization.
- Meteorology: Phase changes of water in the atmosphere are fundamental to weather patterns, cloud formation, and precipitation.
- Food Preservation: Freezing utilizes the enthalpy of fusion to preserve food by lowering its temperature and slowing down microbial growth.
Advanced Concepts and Considerations
While this discussion covers the fundamental aspects of phase change properties, several advanced concepts warrant mention:
- Metastable States: Substances can exist temporarily in states that are not thermodynamically stable, such as supercooled liquids (liquids below their freezing point) and superheated liquids (liquids above their boiling point).
- Phase Transitions in Mixtures: The phase behavior of mixtures is considerably more complex than that of pure substances and involves concepts like azeotropes and eutectics.
- Non-Equilibrium Phase Transitions: Many real-world phase transitions occur under non-equilibrium conditions, leading to phenomena such as nucleation and crystal growth.
- Critical Phenomena: The behavior of substances near the critical point exhibits unique properties, including critical opalescence and significant changes in thermodynamic properties.
Conclusion
The phase change properties of pure substances are a cornerstone of thermodynamics and have far-reaching implications across numerous scientific and engineering disciplines. Understanding these properties, their underlying thermodynamic principles, and their practical applications is crucial for solving a wide range of problems and developing innovative technologies. This comprehensive guide provides a foundational understanding of these essential concepts, paving the way for further exploration into the fascinating world of phase transitions. By understanding these concepts, engineers and scientists can design efficient systems, optimize processes, and develop new materials with tailored properties. Further research into advanced topics like those mentioned above will unlock even more possibilities and further refine our understanding of these fundamental principles.
Latest Posts
Latest Posts
-
What Is The Scanning Objective On A Microscope
Apr 02, 2025
-
Rate Of Change Positive Or Negative
Apr 02, 2025
-
Activation Energy For The Forward Reaction
Apr 02, 2025
-
Base Excision Repair Vs Mismatch Repair
Apr 02, 2025
-
Is Argon Metal Nonmetal Or Metalloid
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Phase Change Properties Of Pure Substances . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
