How Temperature Affects The Rate Of Diffusion
Muz Play
Mar 29, 2025 · 6 min read
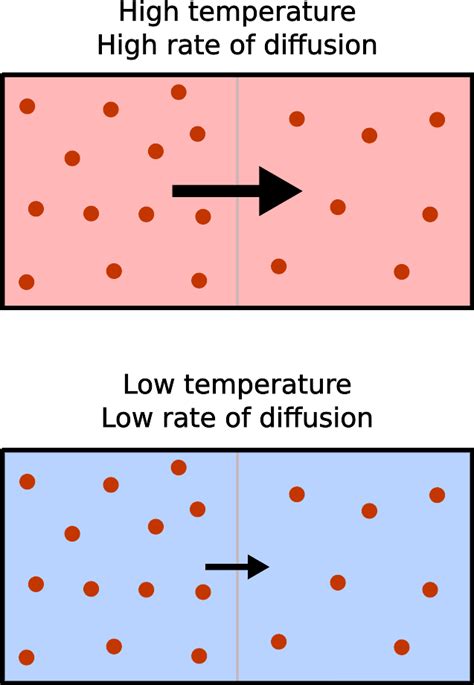
Table of Contents
How Temperature Affects the Rate of Diffusion: A Deep Dive
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in various scientific fields, from biology and chemistry to environmental science and materials engineering. Understanding the factors influencing diffusion is crucial for comprehending numerous phenomena, with temperature being one of the most significant. This article delves into the intricate relationship between temperature and the rate of diffusion, exploring the underlying mechanisms and practical implications across different disciplines.
The Kinetic Molecular Theory and Diffusion
To grasp the influence of temperature on diffusion, we must first consider the kinetic molecular theory (KMT). This theory postulates that matter is composed of tiny particles (atoms or molecules) in constant, random motion. The speed and energy of these particles are directly proportional to the temperature. Higher temperatures equate to greater kinetic energy, resulting in faster and more energetic particle movement.
How Temperature Boosts Kinetic Energy
Imagine a gas confined within a container. At low temperatures, the gas particles possess relatively low kinetic energy; they move slowly and collide infrequently. As the temperature increases, the particles absorb thermal energy, leading to a dramatic increase in their kinetic energy. This increased kinetic energy translates into higher speeds and more frequent, forceful collisions. This heightened activity is the key to understanding why temperature accelerates diffusion.
The Role of Collisions in Diffusion
The random motion of particles, coupled with collisions, is the driving force behind diffusion. Particles constantly collide with each other and with the walls of their container. These collisions are not always perfectly elastic; some energy is transferred during each collision, affecting the direction and speed of the particles. The more frequent and energetic these collisions become (due to higher temperature), the faster the particles spread out, leading to a faster rate of diffusion.
Temperature's Impact on Different States of Matter
The relationship between temperature and diffusion varies slightly depending on the state of matter—solid, liquid, or gas.
Diffusion in Gases: The Fastest Movers
Gases exhibit the highest rate of diffusion due to the large distances between their particles and their relatively weak intermolecular forces. Even at low temperatures, gas particles move rapidly, but an increase in temperature significantly amplifies their movement, drastically increasing the diffusion rate. This is why the smell of perfume or cooking spreads quickly throughout a room, and why gases readily mix with each other. The effect of temperature is particularly pronounced here; a small temperature increase can lead to a substantial increase in diffusion rate.
Diffusion in Liquids: A Moderate Pace
Liquids exhibit slower diffusion rates compared to gases because their particles are closer together, experiencing stronger intermolecular forces. These forces restrict the freedom of movement of the particles. However, increasing the temperature still accelerates diffusion in liquids. The higher kinetic energy overcomes the intermolecular forces more effectively, allowing for quicker movement and spreading of particles. This is observable in the rate at which sugar dissolves in hot water versus cold water. Hot water facilitates faster dissolution because the increased kinetic energy allows sugar molecules to disperse among water molecules more rapidly.
Diffusion in Solids: A Slow and Steady Process
Solids exhibit the slowest diffusion rates. The particles in a solid are tightly packed and strongly bonded to each other, severely restricting their movement. Diffusion in solids is a complex process that often involves the movement of atoms or vacancies (empty lattice sites) within the crystalline structure. While temperature significantly affects diffusion in solids, the effect is generally less dramatic than in gases and liquids. Increasing the temperature provides the energy needed to overcome the strong intermolecular forces, enabling particle movement and diffusion, albeit at a slow pace. This is why many solid-state processes, such as metal annealing or semiconductor doping, are performed at elevated temperatures.
Mathematical Representation of Diffusion and Temperature
The relationship between diffusion and temperature can be mathematically described using Fick's First Law of Diffusion and the Arrhenius equation.
Fick's First Law
Fick's First Law describes the diffusive flux (J) – the amount of substance diffusing per unit area per unit time – as proportional to the concentration gradient (dC/dx). The proportionality constant is the diffusion coefficient (D), which is highly temperature-dependent. A higher temperature results in a larger diffusion coefficient, implying faster diffusion.
J = -D (dC/dx)
The Arrhenius Equation
The Arrhenius equation provides a more comprehensive description of the temperature dependence of the diffusion coefficient:
D = D₀ * exp(-Ea/RT)
Where:
- D is the diffusion coefficient
- D₀ is the pre-exponential factor (related to the frequency of atomic jumps)
- Ea is the activation energy (the energy barrier that must be overcome for diffusion to occur)
- R is the gas constant
- T is the absolute temperature (in Kelvin)
This equation reveals the exponential relationship between the diffusion coefficient and temperature. A small increase in temperature can cause a significant increase in the diffusion coefficient, leading to a rapid increase in the rate of diffusion. The activation energy (Ea) plays a crucial role, representing the energy required to initiate diffusion; a higher activation energy signifies a stronger dependence on temperature.
Practical Applications and Examples
The effect of temperature on diffusion has far-reaching consequences in various fields:
Biology and Medicine:
- Drug delivery: The rate at which drugs diffuse across cell membranes is highly temperature-dependent. Controlled temperature conditions are critical for optimizing drug absorption and distribution.
- Enzyme activity: Enzyme-catalyzed reactions rely on the diffusion of substrate molecules to the active sites of enzymes. Temperature influences both the enzyme's activity and the diffusion rate, affecting reaction rates.
- Oxygen transport in blood: The diffusion of oxygen from the lungs into the bloodstream is affected by temperature. Changes in body temperature can influence oxygen delivery to tissues.
Environmental Science:
- Pollutant dispersion: The spread of pollutants in the atmosphere and water bodies is governed by diffusion. Temperature variations influence the rate at which pollutants disperse, impacting air and water quality.
- Nutrient cycling: The diffusion of nutrients in soil and water systems is crucial for plant growth and ecosystem health. Temperature influences the rate of nutrient diffusion and availability.
Materials Science and Engineering:
- Heat treatments: Heat treatments in metallurgy rely on diffusion processes at elevated temperatures to alter the microstructure and properties of materials.
- Semiconductor fabrication: Diffusion plays a crucial role in the fabrication of semiconductor devices. Controlled temperature is crucial for achieving precise doping profiles.
- Polymer processing: Diffusion influences the properties of polymers during processing, such as blending, mixing, and shaping. Temperature control is vital in optimizing these processes.
Conclusion
Temperature exerts a profound influence on the rate of diffusion. The fundamental principles of the kinetic molecular theory, elucidated through Fick's First Law and the Arrhenius equation, provide a robust framework for understanding this relationship. The exponential dependence of diffusion on temperature is particularly significant, leading to dramatic changes in diffusion rates even with small temperature variations. This understanding has far-reaching consequences in diverse fields, impacting processes ranging from biological systems to materials engineering. Precise control and manipulation of temperature is crucial for optimizing many technological processes and ensuring the efficient functioning of natural systems. Further research and advancements in our understanding of diffusion will continue to have profound implications for scientific and technological progress.
Latest Posts
Latest Posts
-
What Are The Differences Between The Pulmonary And Systemic Circulation
Mar 31, 2025
-
A Perfectly Elastic Supply Curve Is
Mar 31, 2025
-
Do Acids And Bases Conduct Electricity
Mar 31, 2025
-
Inverse Relations And Functions Quick Check
Mar 31, 2025
-
Table Salt Is A Pure Substance
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Temperature Affects The Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
