How To Add Nh2 To A Benzene Ring
Muz Play
Mar 19, 2025 · 5 min read
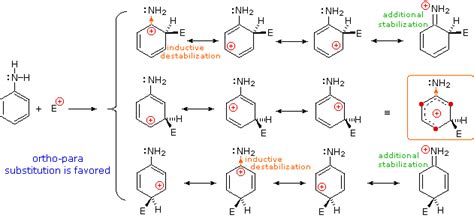
Table of Contents
How to Add NH2 to a Benzene Ring: A Comprehensive Guide to Amination
Adding an amino group (-NH2) to a benzene ring, a process known as amination, is a fundamental reaction in organic chemistry with widespread applications in the synthesis of pharmaceuticals, dyes, and other fine chemicals. This comprehensive guide explores various methods for achieving this transformation, detailing their mechanisms, advantages, limitations, and practical considerations.
Understanding the Benzene Ring and the Amino Group
Before delving into the methods, let's briefly review the properties of the benzene ring and the amino group that influence the reaction pathways. The benzene ring, a highly stable six-carbon aromatic system, is characterized by its delocalized pi electrons, making it relatively unreactive towards electrophilic attack compared to aliphatic systems. The amino group (-NH2), on the other hand, is a strong electron-donating group, significantly influencing the reactivity and properties of the resulting aniline (aminobenzene).
Key Methods for Amination of Benzene
Several methods exist for introducing an amino group onto a benzene ring. Each method has its own merits and drawbacks, making the choice of method dependent on the specific substrate and desired outcome. The most common methods include:
1. Nitration Followed by Reduction: A Classic Approach
This is a widely used and robust method involving two steps:
Step 1: Nitration
The benzene ring is first treated with a mixture of concentrated nitric acid (HNO3) and sulfuric acid (H2SO4) to introduce a nitro group (-NO2). This is an electrophilic aromatic substitution reaction where the nitronium ion (NO2+) acts as the electrophile, attacking the electron-rich benzene ring.
Step 2: Reduction
The nitro group is then reduced to an amino group (-NH2) using a suitable reducing agent. Common reducing agents include:
- Tin (Sn) and hydrochloric acid (HCl): This is a classic method, providing good yields, but generates significant waste.
- Iron (Fe) and hydrochloric acid (HCl): Similar to tin/HCl, it's effective but produces substantial waste.
- Hydrogenation with a catalyst (e.g., Pd/C, Pt/C, Raney Nickel): This is a cleaner and more environmentally friendly method, typically carried out under pressure with hydrogen gas. It offers high selectivity and avoids the generation of metal salts as byproducts.
Mechanism: The reduction process typically involves several steps, including the formation of intermediate hydroxylamines and nitroso compounds before ultimately yielding the aniline.
Advantages: This method is well-established, relatively inexpensive, and offers good yields for a variety of substrates.
Limitations: The use of strong acids and potentially hazardous reducing agents requires careful handling and disposal. The reaction conditions can also be harsh, potentially leading to side reactions.
2. Direct Amination via the Buchwald-Hartwig Amination
This powerful palladium-catalyzed cross-coupling reaction allows for the direct amination of aryl halides (Ar-X, where X is a halide such as Cl, Br, or I). The reaction utilizes a palladium catalyst, a ligand (often a phosphine ligand), a base (e.g., NaOtBu, Cs2CO3), and an amine source (e.g., ammonia, primary or secondary amines).
Mechanism: The reaction proceeds through oxidative addition of the aryl halide to the palladium catalyst, followed by amine addition, reductive elimination, and regeneration of the catalyst.
Advantages: The Buchwald-Hartwig amination offers high selectivity, enabling the direct introduction of diverse amino groups onto aryl halides under relatively mild conditions. It avoids the harsh conditions associated with nitration-reduction methods.
Limitations: The reaction requires a palladium catalyst, which can be expensive. The choice of ligand, base, and reaction conditions is crucial for achieving optimal yields. Sterically hindered substrates might react slower or give lower yields.
3. Hofmann-Martius Rearrangement
This rearrangement reaction converts N-alkyl-N-phenylanilines to the corresponding p-alkylanilines. It involves heating the N-alkyl-N-phenylaniline in the presence of a strong acid, typically sulfuric acid.
Mechanism: The reaction involves a complex series of steps involving protonation, rearrangement, and deprotonation. The alkyl group migrates from the nitrogen atom to the para position of the benzene ring.
Advantages: This method provides access to p-alkylanilines, which are valuable building blocks in organic synthesis.
Limitations: The reaction is specific to N-alkyl-N-phenylanilines and doesn't directly introduce an -NH2 group onto an unsubstituted benzene ring. The reaction conditions are relatively harsh.
4. Gabriel Synthesis (for specific cases)
While not directly applicable to the amination of benzene itself, the Gabriel synthesis is relevant when considering the introduction of an amino group onto other aromatic systems or when preparing specific amines. It utilizes potassium phthalimide as a protected amine source, reacting with alkyl halides followed by hydrazinolysis to release the primary amine. Its application to benzene derivatives might involve using an aryl halide as the starting material, but it’s generally less common for direct benzene amination compared to the methods above.
Choosing the Right Method: Factors to Consider
The selection of the most appropriate method for adding an NH2 group to a benzene ring depends on several factors:
- Substituents on the benzene ring: The presence of other substituents can influence the reactivity and regioselectivity of the reaction. Electron-donating groups activate the ring towards electrophilic aromatic substitution, while electron-withdrawing groups deactivate it.
- Desired regiochemistry: The position of the amino group on the benzene ring (ortho, meta, or para) can be controlled by choosing the appropriate method and reaction conditions.
- Cost and availability of reagents: Some methods are more expensive than others, depending on the catalysts and reagents required.
- Environmental impact: The use of environmentally friendly reagents and reaction conditions is increasingly important.
Safety Precautions
Working with strong acids, reducing agents, and potentially hazardous catalysts requires stringent adherence to safety protocols:
- Proper personal protective equipment (PPE): Always wear appropriate safety goggles, gloves, and lab coats.
- Well-ventilated area: Conduct reactions in a well-ventilated fume hood to avoid inhaling harmful fumes.
- Careful handling of chemicals: Handle all chemicals with care, following proper safety data sheets (SDS).
- Waste disposal: Dispose of all waste chemicals according to proper guidelines.
Conclusion
Adding an amino group to a benzene ring is a crucial transformation in organic synthesis. This guide has explored several key methods, providing insights into their mechanisms, advantages, limitations, and practical considerations. The choice of method depends heavily on the specific context, requiring careful consideration of the factors discussed above. Understanding these methods allows chemists to efficiently and safely synthesize a vast array of aniline derivatives, underpinning advancements across diverse chemical industries. Always prioritize safety and environmental consciousness in experimental procedures.
Latest Posts
Latest Posts
-
Ce Qui Ce Que Ce Dont
Mar 19, 2025
-
Lab Report Titration Of Acids And Bases
Mar 19, 2025
-
Is Lustrous A Metal Or Nonmetal
Mar 19, 2025
-
Difference Between Sn1 Reaction And Sn2 Reaction
Mar 19, 2025
-
The Cellular Organelle Responsible For Protein Synthesis Is
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How To Add Nh2 To A Benzene Ring . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
