Difference Between Sn1 Reaction And Sn2 Reaction
Muz Play
Mar 19, 2025 · 6 min read
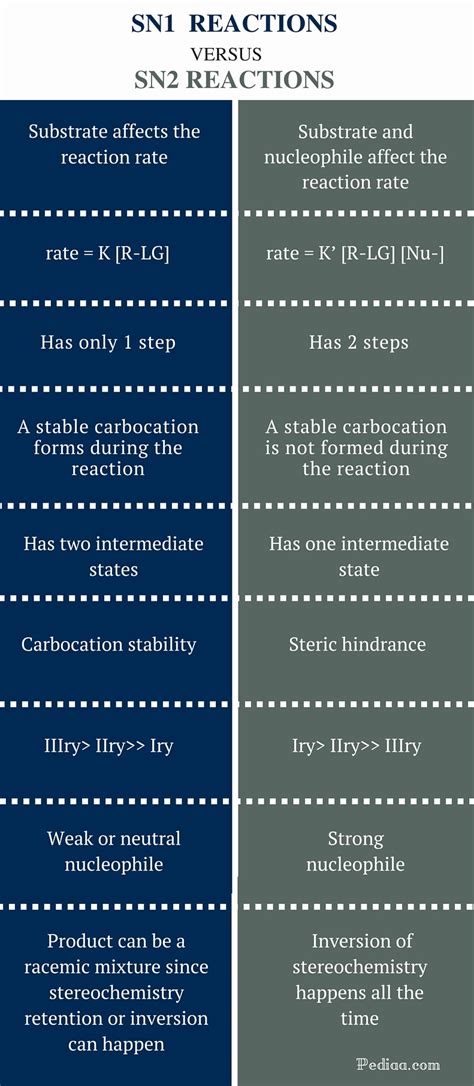
Table of Contents
Unveiling the Nuances: SN1 vs. SN2 Reactions in Organic Chemistry
Organic chemistry, a field teeming with fascinating reactions, often presents students with a seemingly insurmountable challenge: distinguishing between seemingly similar mechanisms. Among the most crucial distinctions to grasp are those between SN1 and SN2 reactions, both of which involve nucleophilic substitution. While both result in the substitution of one nucleophile for another, their mechanisms, kinetics, stereochemistry, and reaction conditions differ significantly. This comprehensive guide will dissect these differences, empowering you to confidently identify and predict the outcome of these crucial reactions.
Understanding Nucleophilic Substitution Reactions
Before diving into the specifics of SN1 and SN2, let's establish a foundational understanding of nucleophilic substitution reactions. These reactions involve the replacement of a leaving group (usually a halide, tosylate, or mesylate) on a carbon atom by a nucleophile – an electron-rich species seeking a positive charge or electron-deficient center. The carbon atom bearing the leaving group is typically sp<sup>3</sup> hybridized.
SN1 Reactions: A Step-by-Step Breakdown
SN1 reactions, or substitution nucleophilic unimolecular reactions, proceed through a two-step mechanism:
Step 1: Ionization
The crucial first step involves the unimolecular ionization of the substrate. The leaving group departs, forming a carbocation intermediate. This step is the rate-determining step, meaning its speed dictates the overall reaction rate. The stability of the carbocation is paramount in determining the reaction's feasibility and speed. Tertiary carbocations are significantly more stable than secondary, which are, in turn, more stable than primary carbocations. Methyl carbocations are extremely unstable.
Key Characteristics of Step 1:
- Unimolecular: Only the substrate participates in this step.
- Rate-determining: The slowest step, and therefore governs the overall reaction rate.
- Carbocation Formation: Formation of a planar, positively charged carbon atom.
Step 2: Nucleophilic Attack
Once the carbocation is formed, the nucleophile attacks the positively charged carbon atom. This attack can occur from either side of the planar carbocation, leading to a mixture of stereoisomers (discussed further in the stereochemistry section). This step is generally fast and not rate-determining.
Key Characteristics of Step 2:
- Bimolecular: Both the carbocation and the nucleophile participate.
- Fast step: Not rate-determining.
- Racemization: Leads to a mixture of stereoisomers (if chiral centers are involved).
SN2 Reactions: A Concerted Mechanism
Unlike SN1 reactions, SN2 reactions, or substitution nucleophilic bimolecular reactions, proceed through a concerted mechanism. This means the bond breaking and bond forming occur simultaneously in a single step.
The Concerted Step
The nucleophile attacks the carbon atom bearing the leaving group from the backside, opposite to the leaving group. This backside attack leads to an inversion of configuration at the stereocenter. Simultaneously, the leaving group departs. The transition state involves a five-coordinate carbon atom with partial bonds to both the nucleophile and the leaving group.
Key Characteristics of the Concerted Step:
- Bimolecular: Both the substrate and the nucleophile are involved in the rate-determining step.
- Concerted: Bond breaking and bond formation happen at the same time.
- Backside Attack: Nucleophile attacks from the opposite side of the leaving group.
Key Differences Between SN1 and SN2 Reactions: A Comparative Table
| Feature | SN1 Reaction | SN2 Reaction |
|---|---|---|
| Mechanism | Two-step (ionization, nucleophilic attack) | Concerted (one-step) |
| Rate Law | Rate = k[substrate] | Rate = k[substrate][nucleophile] |
| Rate-Determining Step | Ionization of the substrate | Nucleophilic attack on the substrate |
| Substrate | Tertiary > secondary > primary (methyl is very slow) | Methyl > primary > secondary (tertiary is very slow) |
| Leaving Group | Good leaving group required | Good leaving group required |
| Nucleophile | Weak or strong nucleophile can be used | Strong nucleophile is preferred |
| Solvent | Polar protic solvent preferred | Polar aprotic solvent preferred |
| Stereochemistry | Racemization (loss of chirality) | Inversion of configuration (Walden Inversion) |
| Carbocation Intermediate | Yes | No |
Factors Affecting SN1 and SN2 Reactions
Several factors influence whether a reaction will proceed via an SN1 or SN2 mechanism:
1. Substrate Structure
- SN1: Tertiary alkyl halides are favored substrates due to the stability of the resulting tertiary carbocation.
- SN2: Primary alkyl halides are preferred because they lack steric hindrance, allowing for the backside attack of the nucleophile. Secondary alkyl halides can participate in both SN1 and SN2.
2. Leaving Group Ability
Both SN1 and SN2 reactions require a good leaving group. Good leaving groups are weak bases, such as halide ions (I<sup>-</sup> > Br<sup>-</sup> > Cl<sup>-</sup> > F<sup>-</sup>), tosylates, and mesylates.
3. Nucleophile Strength and Steric Hindrance
- SN1: The nucleophile's strength is less critical because the rate-determining step doesn't involve it.
- SN2: A strong nucleophile is required because it participates in the rate-determining step. Bulky nucleophiles are less effective due to steric hindrance.
4. Solvent Effects
- SN1: Polar protic solvents are favored because they stabilize the carbocation intermediate through solvation.
- SN2: Polar aprotic solvents are preferred. They solvate the cation but leave the nucleophile relatively unsolvated, making it more reactive.
Stereochemistry: A Crucial Difference
The stereochemical outcomes of SN1 and SN2 reactions represent a fundamental distinction:
- SN1: Leads to racemization if the substrate is chiral. The planar carbocation is attacked equally from both sides, resulting in a mixture of enantiomers.
- SN2: Results in inversion of configuration (Walden inversion). The nucleophile attacks from the backside, pushing the leaving group out and inverting the stereochemistry at the carbon atom.
Predicting the Mechanism: A Practical Approach
Predicting whether a given reaction will follow an SN1 or SN2 pathway requires careful consideration of all the factors mentioned above. Generally, you should analyze the following in this order:
- Substrate: Is it primary, secondary, or tertiary?
- Nucleophile: Is it strong or weak? Is it bulky or unhindered?
- Leaving Group: Is it a good leaving group?
- Solvent: Is it polar protic or polar aprotic?
By systematically assessing these factors, you can make a reasonable prediction about the dominant reaction mechanism. Remember that some reactions may exhibit a mixture of SN1 and SN2 character, especially with secondary substrates.
Examples and Applications
SN1 and SN2 reactions are ubiquitous in organic synthesis, finding application in diverse areas:
- Synthesis of pharmaceuticals: Many drug molecules are synthesized via SN1 or SN2 reactions.
- Polymer chemistry: SN2 reactions play a crucial role in the synthesis of various polymers.
- Natural product synthesis: These reactions are essential in constructing complex molecules found in nature.
- Industrial processes: Numerous industrial processes utilize SN1 and SN2 reactions for manufacturing various chemicals.
Conclusion: Mastering the Nuances of SN1 and SN2
Understanding the intricate differences between SN1 and SN2 reactions is crucial for mastering organic chemistry. By carefully considering the substrate structure, nucleophile strength, leaving group ability, and solvent effects, you can predict the reaction pathway and, consequently, the product(s) formed. This knowledge empowers you to design effective synthetic strategies and navigate the complexities of organic chemical transformations. Remember, practice is key! Working through numerous examples will solidify your understanding and build your confidence in predicting and explaining the outcomes of these fundamental reactions.
Latest Posts
Latest Posts
-
Energy Produced From The Movement Of Particles In A Substance
Mar 19, 2025
-
Can A Strong Acid And Weak Base Be A Buffer
Mar 19, 2025
-
What Points Is Velocity The Highest
Mar 19, 2025
-
Is Salt And Water A Mixture Or A Solution
Mar 19, 2025
-
Find The Matrix Of Linear Transformation
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Sn1 Reaction And Sn2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
