Is Salt And Water A Mixture Or A Solution
Muz Play
Mar 19, 2025 · 5 min read
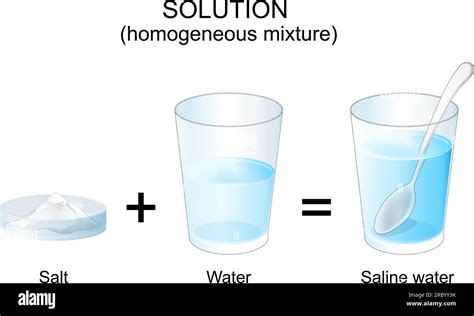
Table of Contents
Is Salt and Water a Mixture or a Solution? A Deep Dive into Chemistry
The seemingly simple question of whether salt water is a mixture or a solution often trips up students and even some seasoned science enthusiasts. While the terms are often used interchangeably, they represent distinct chemical concepts with crucial differences. This in-depth exploration will delve into the fundamental properties of mixtures and solutions, examining the specific case of salt and water to definitively answer the question and highlight the broader implications of understanding these concepts.
Understanding Mixtures and Solutions: Key Differences
Before we analyze salt water, let's establish a clear understanding of mixtures and solutions. Both involve combining two or more substances, but their characteristics differ significantly:
Mixtures: A Heterogeneous Blend
A mixture is a substance composed of two or more components that are physically combined but not chemically bonded. Crucially, the components retain their individual chemical properties and can be separated using physical methods, such as filtration, distillation, or evaporation. Mixtures can be heterogeneous or homogeneous.
-
Heterogeneous Mixtures: These mixtures have a non-uniform composition, meaning the different components are visibly distinct and easily separable. Think of a salad, where you can clearly identify lettuce, tomatoes, and cucumbers. Sand and water is another example; the sand particles remain separate from the water.
-
Homogeneous Mixtures: These mixtures have a uniform composition throughout, meaning the components are evenly distributed at a microscopic level and are not easily distinguishable. Examples include air (a mixture of gases) and sugar dissolved in water (before we get to the salt and water discussion!).
Solutions: A Homogeneous Chemical Combination
A solution is a special type of homogeneous mixture where one substance, the solute, dissolves completely in another substance, the solvent, forming a single, uniform phase. The solute particles are dispersed at a molecular or ionic level, and the resulting solution has a uniform composition throughout. Crucially, the components of a solution are difficult to separate using simple physical methods.
Key characteristics of a solution:
- Uniformity: The solute is evenly distributed throughout the solvent.
- Transparency: Solutions are typically transparent, meaning light can pass through them without significant scattering.
- Filtration Incapability: The solute particles are too small to be separated by filtration.
- No Settling: The solute particles do not settle out over time.
Salt Water: A Detailed Analysis
Now, let's apply these definitions to the salt water example. When table salt (sodium chloride, NaCl) is added to water (H₂O), the ionic bonds in the salt crystal break down. The positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻) are surrounded by water molecules, a process called hydration. These ions become dispersed evenly throughout the water, forming a homogeneous mixture.
Evidence supporting salt water as a solution:
- Uniformity: A well-mixed saltwater solution appears uniform throughout; you cannot visually distinguish salt from water.
- Transparency: Saltwater solutions are transparent, especially at low concentrations.
- Filtration Ineffectiveness: You cannot separate the salt from the water using a simple filter.
- Non-settling: The salt ions remain dispersed in the water; they don't settle to the bottom.
- Chemical Changes: Although no new chemical substance is formed (it remains NaCl and H2O), the interaction between the water molecules and the salt ions represent a chemical change. Water molecules are polar, meaning they have a positive and negative end. The positive ends of the water molecules are attracted to the negatively charged chloride ions, and the negative ends are attracted to the positively charged sodium ions. This interaction disrupts the crystal lattice structure of the salt, facilitating its dissolution.
Therefore, based on these properties, salt water is definitively a solution, not just a mixture. It's a homogeneous mixture, but the specific interactions between the solute (salt) and the solvent (water) and the resulting properties elevate it to the classification of a true solution.
Beyond Salt and Water: Expanding the Understanding
Understanding the difference between mixtures and solutions is crucial in various scientific disciplines, including chemistry, biology, and environmental science. Here are some examples that illustrate the broader application of these concepts:
-
Medicine: Many drugs are administered as solutions, ensuring even distribution and absorption into the body. Understanding the solubility of drugs in various solvents is critical for drug formulation and delivery.
-
Environmental Science: The study of water pollution involves analyzing the solubility of various pollutants in water. Understanding how pollutants dissolve and interact with water is crucial for developing effective remediation strategies.
-
Food Science: Many food products are solutions or suspensions (a type of mixture). The solubility of ingredients in water or other solvents significantly affects texture, taste, and shelf life.
-
Material Science: The properties of many materials depend on their composition, whether they are mixtures or solutions. For example, the strength and durability of alloys are influenced by how the constituent metals are mixed and interact.
-
Geology: Many geological processes involve the dissolution and precipitation of minerals in water. Understanding the solubility of minerals helps scientists interpret geological formations and predict mineral deposits.
Addressing Common Misconceptions
Some common misunderstandings regarding mixtures and solutions need clarification:
-
All solutions are mixtures, but not all mixtures are solutions: This is a crucial point to grasp. All solutions are indeed homogeneous mixtures, but the reverse is not true. Homogeneous mixtures that do not involve complete dissolution at a molecular level are not solutions.
-
Separation methods: While many mixtures can be separated by simple physical methods, solutions require more sophisticated techniques like distillation or chromatography.
-
Particle size: The key distinction between mixtures and solutions often lies in the size of the dispersed particles. In solutions, the solute particles are at the atomic or molecular level, while mixtures can have larger particles.
Conclusion: Salt Water - A Textbook Example of a Solution
In conclusion, the question of whether salt water is a mixture or a solution has a definitive answer: it's a solution. The complete dissolution of salt in water, resulting in a homogeneous, transparent mixture with uniformly dispersed ions, aligns perfectly with the definition of a solution. Understanding this fundamental distinction highlights the broader importance of grasping the concepts of mixtures and solutions in various scientific contexts. The seemingly simple act of dissolving salt in water serves as a potent illustration of the complex chemical interactions that govern the world around us. It’s a perfect example to explore the fundamentals of chemistry, laying a groundwork for more advanced topics and applications. The more we understand these basic concepts, the more equipped we are to tackle complex problems across a range of scientific disciplines.
Latest Posts
Latest Posts
-
Parts Of A Fire Hydrant Diagram
Mar 19, 2025
-
What Is The Ion Product Constant For Water
Mar 19, 2025
-
Strong Acid And Strong Base Reaction
Mar 19, 2025
-
What Is The Period Of A Cosine Function
Mar 19, 2025
-
Elements In An Element Family Have Similar
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Salt And Water A Mixture Or A Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
