What Is The Ion Product Constant For Water
Muz Play
Mar 19, 2025 · 6 min read
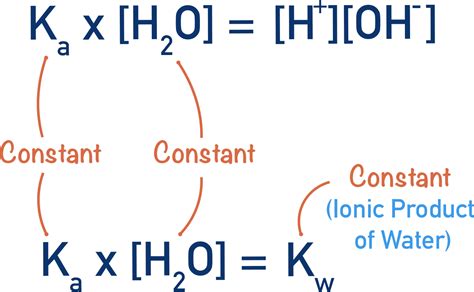
Table of Contents
What is the Ion Product Constant for Water (Kw)? A Deep Dive
The ion product constant for water, denoted as K<sub>w</sub>, is a fundamental concept in chemistry that describes the self-ionization of water molecules. Understanding K<sub>w</sub> is crucial for comprehending various chemical processes, particularly acid-base equilibria and pH calculations. This comprehensive guide delves into the intricacies of K<sub>w</sub>, exploring its definition, significance, temperature dependence, and applications in various fields.
Understanding the Self-Ionization of Water
Water, despite its seemingly simple structure (H₂O), exhibits a fascinating property: self-ionization. This means that water molecules can spontaneously react with each other, transferring a proton (H⁺) from one molecule to another. This process can be represented by the following equilibrium reaction:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equation shows that two water molecules react to form a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). The hydronium ion is a hydrated proton, representing the proton's interaction with surrounding water molecules. While often simplified to H⁺, using H₃O⁺ provides a more accurate representation of the proton's existence in aqueous solutions.
Defining the Ion Product Constant (Kw)
The ion product constant for water (K<sub>w</sub>) is the equilibrium constant for the self-ionization reaction. It's defined as the product of the concentrations of hydronium and hydroxide ions:
K<sub>w</sub> = [H₃O⁺][OH⁻]
At 25°C (298 K), the value of K<sub>w</sub> is approximately 1.0 × 10⁻¹⁴. This means that in pure water at this temperature, the concentration of both hydronium and hydroxide ions is 1.0 × 10⁻⁷ M. This seemingly small value highlights the fact that water is a weak electrolyte—only a tiny fraction of water molecules ionize at any given time.
The Significance of Kw = 1.0 x 10⁻¹⁴ at 25°C
This specific value at 25°C is extremely important because it forms the foundation for calculating pH and pOH. Since [H₃O⁺] = [OH⁻] in pure water, we can derive:
[H₃O⁺] = [OH⁻] = √K<sub>w</sub> = √(1.0 × 10⁻¹⁴) = 1.0 × 10⁻⁷ M
This allows us to define pH and pOH:
- pH = -log[H₃O⁺]
- pOH = -log[OH⁻]
In pure water at 25°C, both pH and pOH are equal to 7, indicating a neutral solution.
The Temperature Dependence of Kw
It's crucial to remember that K<sub>w</sub> is temperature-dependent. As the temperature increases, the degree of water self-ionization increases, leading to a higher K<sub>w</sub> value. This is because higher temperatures provide more energy for the water molecules to overcome the energy barrier required for the self-ionization reaction. The relationship is not linear; the increase in K<sub>w</sub> is more pronounced at higher temperatures.
Here's a table illustrating the change in K<sub>w</sub> with temperature:
| Temperature (°C) | Kw |
|---|---|
| 0 | 0.114 × 10⁻¹⁴ |
| 10 | 0.292 × 10⁻¹⁴ |
| 20 | 0.681 × 10⁻¹⁴ |
| 25 | 1.00 × 10⁻¹⁴ |
| 30 | 1.47 × 10⁻¹⁴ |
| 40 | 2.92 × 10⁻¹⁴ |
| 50 | 5.47 × 10⁻¹⁴ |
This temperature dependence highlights that the neutral pH of 7 at 25°C is not a universal constant; it changes with temperature.
Kw and Acid-Base Equilibria
The ion product constant plays a vital role in understanding acid-base equilibria. Acids increase the concentration of H₃O⁺ ions, while bases increase the concentration of OH⁻ ions. The relationship between [H₃O⁺] and [OH⁻] always remains consistent with K<sub>w</sub>:
[H₃O⁺][OH⁻] = K<sub>w</sub>
This equation allows us to calculate the concentration of either hydronium or hydroxide ions if the concentration of the other is known, regardless of whether the solution is acidic, basic, or neutral.
Calculating pH and pOH in Acidic and Basic Solutions
Using K<sub>w</sub>, we can calculate pH and pOH in solutions where acids or bases have been added. For example, if we have a solution with a known [H₃O⁺], we can calculate [OH⁻] using:
[OH⁻] = K<sub>w</sub> / [H₃O⁺]
Then, we can use the standard formulas to calculate pH and pOH. Similarly, if we know [OH⁻], we can calculate [H₃O⁺] and then pH and pOH.
Applications of Kw
The ion product constant for water finds applications in various areas:
-
Environmental Science: Understanding K<sub>w</sub> is crucial for analyzing water quality. The pH of water bodies significantly impacts aquatic life and overall ecosystem health. Measuring pH allows for assessing the acidity or basicity of water samples and identifying potential pollutants.
-
Medicine and Biology: pH plays a critical role in biological systems. Maintaining a specific pH range is vital for enzyme activity, cellular function, and overall physiological processes. K<sub>w</sub> helps in understanding and managing pH imbalances in the human body.
-
Chemical Engineering: In industrial processes involving aqueous solutions, controlling pH is often essential. K<sub>w</sub> is used in designing and optimizing chemical reactions, ensuring the desired reaction conditions are maintained.
-
Analytical Chemistry: K<sub>w</sub> is fundamental in many analytical techniques, such as titrations, where pH changes are used to determine the concentration of unknown solutions.
Advanced Concepts and Considerations
While the simple equation K<sub>w</sub> = [H₃O⁺][OH⁻] provides a good understanding of the concept, more sophisticated approaches are needed for highly accurate calculations, especially in non-ideal solutions. These include:
-
Activity coefficients: In concentrated solutions, the interactions between ions affect their effective concentrations (activities). Activity coefficients are used to correct for these deviations from ideal behavior.
-
Ionic strength: The ionic strength of a solution, which reflects the total concentration of ions, influences the activity coefficients.
-
Temperature and Pressure: Precise calculations require considering the variations in K<sub>w</sub> with both temperature and pressure, especially in extreme conditions.
Conclusion
The ion product constant for water (K<sub>w</sub>) is a seemingly simple yet powerful concept that underpins our understanding of aqueous solutions and their behavior. Its temperature dependence, application in acid-base equilibria, and role in various scientific disciplines highlight its importance. Mastering K<sub>w</sub> is essential for anyone studying chemistry, biochemistry, environmental science, or related fields. Understanding the nuances, including the effect of temperature and the use of activity coefficients, provides a deeper and more accurate understanding of this fundamental constant. By grasping the fundamental principles and the more advanced considerations, one can accurately predict and interpret the behavior of aqueous solutions in a wide range of applications.
Latest Posts
Latest Posts
-
Is Alcohol A Acid And A Base Bronsted
Mar 19, 2025
-
Do Acids Gain Or Lose Hydrogen Ions
Mar 19, 2025
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ion Product Constant For Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
