Lewis Diagram For A Ion With A Total Of Electrons
Muz Play
Mar 19, 2025 · 6 min read
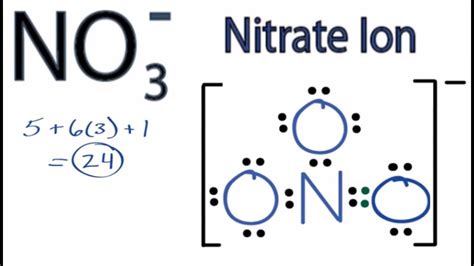
Table of Contents
- Lewis Diagram For A Ion With A Total Of Electrons
- Table of Contents
- Lewis Diagrams for Ions: A Comprehensive Guide
- Understanding Valence Electrons and Ionic Bonds
- Valence Electrons: The Key Players
- Ionic Bonds: A Transfer of Electrons
- Determining the Total Number of Electrons in an Ion
- Drawing Lewis Diagrams for Ions: Step-by-Step
- Examples of Lewis Diagrams for Ions
- Example 1: Ammonium Ion (NH₄⁺)
- Example 2: Nitrate Ion (NO₃⁻)
- Example 3: Sulfate Ion (SO₄²⁻)
- Exceptions to the Octet Rule
- Importance of Lewis Diagrams in Chemistry
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Lewis Diagrams for Ions: A Comprehensive Guide
Lewis diagrams, also known as Lewis structures or electron dot diagrams, are essential tools in chemistry for visualizing the valence electrons of atoms and molecules. They are particularly useful for understanding the bonding in ions, charged species formed by the gain or loss of electrons. This comprehensive guide will delve into the intricacies of drawing Lewis diagrams for ions, covering various scenarios and providing step-by-step instructions. We'll explore how to determine the total number of electrons, account for charges, and represent different types of ionic bonds.
Understanding Valence Electrons and Ionic Bonds
Before diving into drawing Lewis diagrams for ions, let's establish a firm understanding of fundamental concepts.
Valence Electrons: The Key Players
Valence electrons are the outermost electrons in an atom. These electrons are the ones involved in chemical bonding. The number of valence electrons determines an atom's reactivity and the type of bonds it can form. You can typically determine the number of valence electrons by looking at the group number (or column) of the element on the periodic table. For example, elements in Group 1 (alkali metals) have one valence electron, Group 17 (halogens) have seven, and Group 18 (noble gases) have eight (except for helium, which has two).
Ionic Bonds: A Transfer of Electrons
Ionic bonds form when one atom transfers one or more valence electrons to another atom. This transfer results in the formation of ions: positively charged cations (formed by electron loss) and negatively charged anions (formed by electron gain). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. The driving force behind ionic bond formation is the achievement of a stable electron configuration, often resembling that of a noble gas (octet rule).
Determining the Total Number of Electrons in an Ion
Accurately determining the total number of electrons is crucial for drawing a correct Lewis diagram for an ion. Here's how:
-
Identify the constituent atoms: Begin by identifying all the atoms present in the ion.
-
Determine the number of valence electrons for each atom: Use the periodic table to find the number of valence electrons each atom contributes.
-
Account for the charge: This is the critical step. For cations (positive ions), subtract the magnitude of the charge from the total number of valence electrons. For anions (negative ions), add the magnitude of the charge to the total number of valence electrons. Each positive charge represents a lost electron, and each negative charge represents a gained electron.
Example: Consider the hydroxide ion, OH⁻.
- Oxygen (O) has 6 valence electrons.
- Hydrogen (H) has 1 valence electron.
- The ion has a -1 charge, meaning one extra electron is added.
Total electrons: 6 + 1 + 1 = 8 electrons
Drawing Lewis Diagrams for Ions: Step-by-Step
The process of drawing a Lewis diagram for an ion is similar to that of a neutral molecule, but with crucial adjustments for the charge. Here’s a detailed, step-by-step procedure:
-
Determine the central atom: Generally, the least electronegative atom (except for hydrogen, which is always terminal) is the central atom.
-
Arrange atoms: Place the central atom in the center and surround it with the other atoms.
-
Connect atoms with single bonds: Each bond represents a shared pair of electrons (two electrons).
-
Distribute remaining electrons: Add the remaining electrons as lone pairs (two electrons per pair) to the outer atoms to satisfy the octet rule (eight electrons for most atoms, except hydrogen, which follows the duet rule, needing two electrons).
-
Satisfy the octet rule for the central atom: If the central atom doesn't have an octet, move lone pairs from outer atoms to form double or triple bonds.
-
Enclose the ion in square brackets and indicate the charge: This is essential to show that it's an ion.
Examples of Lewis Diagrams for Ions
Let's illustrate the process with several examples:
Example 1: Ammonium Ion (NH₄⁺)
-
Total electrons: N (5) + 4H (1 each) - 1 (positive charge) = 8 electrons
-
Central atom: Nitrogen (N)
-
Structure: Nitrogen in the center, surrounded by four hydrogen atoms. Each hydrogen is bonded to the nitrogen with a single bond.
-
Final Lewis Diagram: [H-N-H]⁺ | | H H
Example 2: Nitrate Ion (NO₃⁻)
-
Total electrons: N (5) + 3O (6 each) + 1 (negative charge) = 24 electrons
-
Central atom: Nitrogen (N)
-
Structure: Nitrogen in the center, surrounded by three oxygen atoms.
-
Electron distribution: Initial single bonds use 6 electrons, leaving 18. Distribute these as lone pairs on the oxygens, leading to two oxygens with three lone pairs each and one with two lone pairs. However, nitrogen lacks an octet.
-
Octet completion: Move one lone pair from an oxygen to form a double bond with the nitrogen, creating resonance structures.
-
Final Lewis Diagram: The nitrate ion displays resonance; several equivalent structures exist where the double bond can be between the nitrogen and any one of the oxygen atoms. All structures should be shown, indicated by resonance arrows. The final structure will be enclosed in brackets with a -1 charge.
Example 3: Sulfate Ion (SO₄²⁻)
-
Total electrons: S (6) + 4O (6 each) + 2 (negative charge) = 32 electrons
-
Central atom: Sulfur (S)
-
Structure: Sulfur in the center, surrounded by four oxygen atoms.
-
Electron distribution: Initial single bonds use 8 electrons, leaving 24. Distribute these as lone pairs on the oxygens. Sulfur lacks an octet.
-
Octet completion: Move two lone pairs from two different oxygens to form two double bonds with the sulfur.
-
Final Lewis Diagram: The sulfate ion also exhibits resonance, with multiple equivalent structures. All structures should be included, and the ion should be enclosed in brackets with a 2- charge.
Exceptions to the Octet Rule
While the octet rule provides a useful guideline, there are exceptions:
-
Electron-deficient molecules: Some molecules, like boron trifluoride (BF₃), have fewer than eight electrons around the central atom.
-
Expanded octets: Elements in the third period and beyond can accommodate more than eight electrons due to the availability of d orbitals. Examples include phosphorus pentachloride (PCl₅) and sulfur hexafluoride (SF₆).
-
Odd-electron molecules: Molecules with an odd number of valence electrons, like nitrogen dioxide (NO₂), cannot satisfy the octet rule for all atoms.
Drawing Lewis diagrams for ions that fall under these exceptions requires careful consideration of these factors.
Importance of Lewis Diagrams in Chemistry
Lewis diagrams are fundamental tools for:
-
Predicting molecular geometry: The arrangement of atoms and lone pairs influences the molecular shape.
-
Understanding chemical reactivity: The presence of lone pairs or unfilled octets can indicate reactive sites.
-
Explaining bonding: They clearly show the distribution of electrons in bonds and lone pairs.
-
Visualizing resonance structures: For many ions and molecules, resonance structures provide a more accurate representation of the bonding.
Conclusion
Mastering the art of drawing Lewis diagrams for ions is crucial for a thorough understanding of chemical bonding and the behavior of ionic compounds. By following the steps outlined in this guide and carefully considering the total number of electrons and any exceptions to the octet rule, you can confidently represent the electronic structure of various ions, laying a strong foundation for further exploration of chemical concepts. Remember to practice regularly, using various examples to solidify your understanding. The more you practice, the easier it will become to visualize and draw accurate Lewis diagrams for any ion.
Latest Posts
Latest Posts
-
How Do You Calculate The Rate Of Diffusion
Mar 21, 2025
-
What Is A Unique Property Of Water
Mar 21, 2025
-
In A Phospholipid Molecule The Head Is
Mar 21, 2025
-
How To Find The Coefficient Chemistry Calculus
Mar 21, 2025
-
How To Calculate The Temperature Change
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Lewis Diagram For A Ion With A Total Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
