In A Phospholipid Molecule The Head Is
Muz Play
Mar 21, 2025 · 7 min read
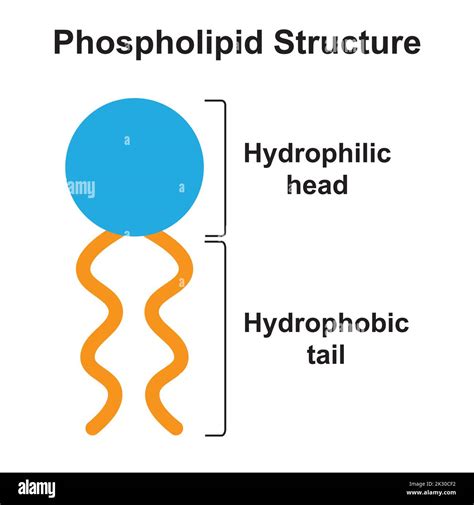
Table of Contents
In a Phospholipid Molecule, the Head Is… Hydrophilic! Understanding Phospholipid Structure and Function
Phospholipids are fundamental components of cell membranes, forming the crucial barrier that separates the internal cellular environment from the external world. Understanding their structure is key to understanding how cells function. The core question, "In a phospholipid molecule, the head is...", leads us into the fascinating world of amphipathic molecules and their role in biological systems. This article delves deep into the structure of phospholipids, exploring the properties of the hydrophilic head, the hydrophobic tails, and how these contrasting characteristics drive the formation of the lipid bilayer—the foundation of all cellular life.
The Phospholipid Bilayer: A Tale of Two Tails (and One Head)
Before we dive into the specifics of the head, let's establish a foundational understanding of the phospholipid molecule as a whole. A phospholipid is a type of lipid molecule composed of three main components:
- A hydrophilic head: This is the "water-loving" part of the molecule, which interacts readily with water.
- Two hydrophobic tails: These are the "water-fearing" parts of the molecule, which repel water and interact favorably with each other.
- A glycerol backbone: This serves as the connecting scaffold, linking the head group to the fatty acid tails.
This unique arrangement of hydrophilic and hydrophobic components is what makes phospholipids amphipathic. This amphipathic nature is crucial to the formation of the phospholipid bilayer, a double layer of phospholipids that forms the basis of all cell membranes.
Decoding the Hydrophilic Head: Structure and Properties
Now, let's address the central question: In a phospholipid molecule, the head is hydrophilic. This hydrophilic nature stems from its chemical composition. The head group is typically composed of a phosphate group and a polar molecule, often a choline, serine, ethanolamine, or inositol group.
The Phosphate Group: The Charge Carrier
The phosphate group (PO₄³⁻) carries a negative charge, making it highly polar. This charge is crucial for the head group's interaction with water molecules, which are also polar. The negative charge interacts with the partially positive hydrogen atoms of water molecules through electrostatic interactions, creating a strong attraction.
The Polar Molecule: Adding to the Attraction
The polar molecule attached to the phosphate group further enhances the hydrophilic character of the head. For example, in phosphatidylcholine (a common phospholipid), the polar molecule is choline, a positively charged quaternary ammonium ion. This positive charge interacts with the partially negative oxygen atoms of water molecules, further strengthening the hydrophilic interaction. Other polar head groups contribute similar interactions.
The Hydrophobic Tails: A Repulsion of Water
In stark contrast to the hydrophilic head, the two tails of the phospholipid molecule are hydrophobic. These tails are typically composed of fatty acid chains, long hydrocarbon chains with primarily carbon-carbon and carbon-hydrogen bonds. These bonds are nonpolar, meaning they do not have a significant separation of charge.
Nonpolarity and Water Repulsion
The nonpolar nature of the fatty acid tails means they cannot form favorable interactions with water molecules. Water molecules are highly polar and are strongly attracted to each other through hydrogen bonds. The introduction of nonpolar molecules disrupts this intricate hydrogen bonding network, leading to an unfavorable energetic state. To minimize this disruption, the hydrophobic tails cluster together, away from the aqueous environment.
Saturation and Unsaturation: Impact on Fluidity
The degree of saturation of the fatty acid tails significantly impacts the fluidity of the membrane. Saturated fatty acids have no double bonds between carbon atoms, resulting in a straight, tightly packed structure. This leads to a less fluid membrane. Unsaturated fatty acids, on the other hand, contain one or more double bonds, introducing kinks in the fatty acid chains. These kinks prevent tight packing, resulting in a more fluid membrane.
The Amphipathic Nature and Bilayer Formation
The contrasting properties of the hydrophilic head and hydrophobic tails are the driving forces behind the formation of the phospholipid bilayer. When phospholipids are placed in an aqueous environment, they spontaneously self-assemble into a bilayer structure. The hydrophilic heads orient themselves towards the aqueous environment, both internally and externally, while the hydrophobic tails cluster together in the interior of the bilayer, avoiding contact with water.
The Stability of the Bilayer
This arrangement minimizes the energetically unfavorable interactions between water and the hydrophobic tails, maximizing the stability of the system. The result is a stable, self-sealing bilayer, a remarkably robust structure that forms the fundamental boundary of cells.
The Fluid Mosaic Model
The phospholipid bilayer is not a static structure; it's dynamic and fluid. The phospholipids are constantly moving laterally within the plane of the membrane, creating a fluid mosaic. This fluidity allows for the movement of membrane proteins and other molecules within the membrane, facilitating various cellular processes.
Beyond the Basics: Variations in Phospholipid Head Groups
While the core structure of a phospholipid – a hydrophilic head and two hydrophobic tails – remains consistent, variations in the head group lead to different types of phospholipids with diverse functions. These variations impact the membrane's physical properties, influencing its fluidity, permeability, and interactions with other molecules.
- Phosphatidylcholine (PC): The most abundant phospholipid in most cell membranes, characterized by a choline head group. It contributes significantly to membrane fluidity.
- Phosphatidylethanolamine (PE): Another common phospholipid, featuring an ethanolamine head group. It plays a role in membrane curvature and vesicle formation.
- Phosphatidylserine (PS): A negatively charged phospholipid, with a serine head group. Its presence on the outer leaflet of the cell membrane is a signal for apoptosis (programmed cell death).
- Phosphatidylinositol (PI): Plays a critical role in cell signaling and membrane trafficking. It can be phosphorylated to produce various second messengers involved in various cellular processes.
- Phosphatidic acid (PA): A simple phospholipid, with only a phosphate head group. It serves as a precursor for the synthesis of other phospholipids and plays a role in membrane fusion.
The Importance of Phospholipids in Cellular Processes
The phospholipid bilayer is far more than just a passive barrier; it's actively involved in many vital cellular processes. Its role extends far beyond simply separating the cell's interior from its surroundings.
Membrane Fluidity and Protein Function
The fluidity of the membrane, dictated by the phospholipid composition, is crucial for the proper functioning of membrane proteins. These proteins are embedded within the bilayer, and their movement and interaction are influenced by the membrane's fluidity. Changes in membrane fluidity can affect processes like signal transduction, nutrient transport, and cell-cell interactions.
Membrane Trafficking and Vesicle Formation
Phospholipids are essential components of vesicles, small membrane-bound sacs that transport molecules within cells. The curvature of these vesicles is influenced by the types of phospholipids present in their membranes.
Cell Signaling and Signal Transduction
Certain phospholipids, such as phosphatidylinositol, act as precursors for second messengers involved in cell signaling. These second messengers transmit signals from the cell surface to intracellular targets, initiating various cellular responses.
Apoptosis (Programmed Cell Death)
The exposure of phosphatidylserine on the outer leaflet of the cell membrane serves as a crucial signal for apoptosis. This "eat me" signal alerts the immune system to remove the dying cell, preventing inflammation and damage to surrounding tissues.
Conclusion: The Hydrophilic Head and the Living Cell
In a phospholipid molecule, the head is indeed hydrophilic, and this seemingly simple characteristic is fundamental to the existence of life as we know it. The amphipathic nature of phospholipids, driven by the interplay of hydrophilic and hydrophobic interactions, drives the formation of the phospholipid bilayer, the very foundation of cellular membranes. These membranes are not merely barriers; they are dynamic, functional structures involved in countless cellular processes, from nutrient transport to cell signaling and apoptosis. Understanding the intricacies of phospholipid structure and function is therefore crucial for comprehending the fundamental workings of cells and, ultimately, life itself. The exploration of the hydrophilic head, alongside the hydrophobic tails, reveals the elegant simplicity and profound importance of this fundamental building block of life.
Latest Posts
Latest Posts
-
What Is The Mass Number Of An Atom Equal To
Mar 28, 2025
-
Le Chateliers Principle Lab Report Answers
Mar 28, 2025
-
How To Calculate Mass Of Excess Reactant
Mar 28, 2025
-
What Does Newtons First Law Say
Mar 28, 2025
-
Does Ionic Have High Boiling Point
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about In A Phospholipid Molecule The Head Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
