What Is The Mass Number Of An Atom Equal To
Muz Play
Mar 28, 2025 · 6 min read
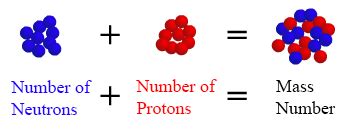
Table of Contents
What is the Mass Number of an Atom Equal To? A Deep Dive into Atomic Structure
Understanding the mass number of an atom is fundamental to grasping the basics of chemistry and physics. This seemingly simple number holds a wealth of information about an atom's composition and properties. This comprehensive guide will explore what the mass number represents, how it's calculated, its relationship to isotopes, and its applications in various scientific fields.
Defining the Mass Number
The mass number (A) of an atom is the total number of protons and neutrons present in its nucleus. It's a crucial characteristic that distinguishes one atom from another. Importantly, the mass number doesn't include electrons because their mass is negligible compared to that of protons and neutrons. Therefore, we can express the mass number as:
A = Z + N
Where:
- A represents the mass number.
- Z represents the atomic number (number of protons).
- N represents the neutron number (number of neutrons).
This simple equation is the cornerstone of understanding atomic mass. It provides a direct link between the fundamental particles within an atom and a measurable quantity – the mass number.
The Role of Protons and Neutrons
To fully appreciate the mass number, we need to understand the roles of protons and neutrons within the atomic nucleus.
Protons: Defining the Element
Protons, carrying a positive charge, determine the atomic number (Z) of an element. This number is unique to each element and dictates its position on the periodic table. Hydrogen (H), with one proton, has an atomic number of 1; Helium (He), with two protons, has an atomic number of 2, and so on. The atomic number essentially defines the chemical identity of an element. All atoms of a particular element have the same number of protons.
Neutrons: Influencing Isotopes
Neutrons, carrying no charge, contribute significantly to the atom's mass but don't affect its chemical properties. The number of neutrons in an atom's nucleus can vary, even within the same element. This variation leads to the existence of isotopes.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This means they have the same atomic number (Z) but different mass numbers (A). For example, carbon-12 (¹²C) has 6 protons and 6 neutrons (A = 12), while carbon-14 (¹⁴C) has 6 protons and 8 neutrons (A = 14). Both are isotopes of carbon, exhibiting similar chemical behavior but differing in their mass and radioactive properties (¹⁴C is radioactive).
Understanding Isotope Notation
Isotopes are commonly represented using the following notation:
A
X
Z
Where:
- X is the chemical symbol of the element.
- A is the mass number.
- Z is the atomic number.
For instance, the notation for carbon-14 is:
¹⁴C
6
This notation concisely conveys the element's identity and the number of protons and neutrons.
Calculating the Mass Number: Examples
Let's illustrate the calculation of the mass number with a few examples:
Example 1: Oxygen-16 (¹⁶O)
Oxygen has an atomic number (Z) of 8 (8 protons). Oxygen-16 has a mass number (A) of 16. Therefore, the neutron number (N) can be calculated:
N = A - Z = 16 - 8 = 8 neutrons
Example 2: Uranium-238 (²³⁸U)
Uranium has an atomic number (Z) of 92. Uranium-238 has a mass number (A) of 238. The neutron number (N) is:
N = A - Z = 238 - 92 = 146 neutrons
Example 3: Determining the Mass Number Given Protons and Neutrons
If an atom has 17 protons and 18 neutrons, its atomic number (Z) is 17, and its neutron number (N) is 18. Therefore, its mass number (A) is:
A = Z + N = 17 + 18 = 35
This atom would be Chlorine-35 (³⁵Cl).
The Mass Number and Atomic Mass: Key Differences
While closely related, the mass number and atomic mass are not interchangeable terms.
- Mass number (A) is the sum of protons and neutrons, always a whole number.
- Atomic mass (or atomic weight) is the average mass of all isotopes of an element, considering their relative abundances. This is a weighted average and is often not a whole number. For example, the atomic mass of chlorine is approximately 35.45 amu (atomic mass units), reflecting the presence of both ³⁵Cl and ³⁷Cl isotopes.
The mass number provides information about a specific isotope, while the atomic mass represents the average mass of all isotopes of an element as they naturally occur.
Applications of Mass Number
The mass number finds applications in diverse scientific fields:
-
Nuclear Physics: Understanding nuclear reactions and radioactive decay requires knowledge of the mass number. In nuclear reactions, the mass number is conserved (though mass may be converted to energy as per Einstein's E=mc²).
-
Nuclear Medicine: Radioisotopes with specific mass numbers are used in diagnostic and therapeutic procedures, utilizing their radioactive properties for imaging or targeted therapy.
-
Chemistry: Isotopic analysis, using mass spectrometry, helps determine the relative abundance of isotopes in a sample, providing insights into various processes. This has implications for fields like geochemistry and forensic science.
-
Materials Science: The mass number influences the properties of materials. Isotopic substitution can be used to study materials properties and design novel materials with specific functionalities.
Mass Number and its Limitations
While the mass number is a valuable concept, it has certain limitations:
-
It doesn't directly represent the mass of the atom: The mass number is an integer approximation of the atomic mass, neglecting the small mass difference between protons and neutrons and the binding energy of the nucleus.
-
It doesn't account for electron mass: The mass of electrons is negligible compared to the mass of protons and neutrons and is therefore not included in the mass number calculation.
-
It doesn't provide information about the atom's chemical behavior: The chemical behavior is solely determined by the atomic number and the electronic configuration, not the mass number.
Conclusion
The mass number (A), being the sum of protons and neutrons in an atom's nucleus, is a fundamental concept in atomic structure. Its calculation provides a direct link between the number of subatomic particles and the atom's mass. Understanding the mass number is crucial for comprehending isotopes, nuclear reactions, and various applications across multiple scientific disciplines. While it's a powerful tool, remembering its limitations and distinguishing it from atomic mass ensures a more nuanced understanding of atomic structure and its implications. The exploration of mass number opens doors to comprehending the vast complexities and wonders of the atomic world. Further exploration into related topics like nuclear binding energy and isotopic abundances will enrich the understanding of the significance of mass number in atomic and nuclear physics.
Latest Posts
Latest Posts
-
The Atomic Mass Is Equal To The Number Of
Mar 31, 2025
-
What Is The Mode Of Nutrition For Fungi
Mar 31, 2025
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Number Of An Atom Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
