The Atomic Mass Is Equal To The Number Of
Muz Play
Mar 31, 2025 · 6 min read
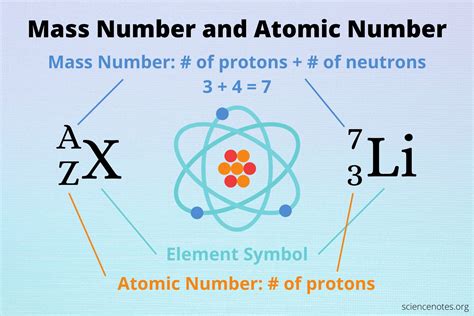
Table of Contents
The Atomic Mass: A Deep Dive into What It Equals and Why It Matters
The atomic mass, also known as atomic weight, is a crucial concept in chemistry and physics. It represents the total mass of an atom, typically expressed in atomic mass units (amu). However, simply stating that atomic mass equals the number of something is an oversimplification. The truth is more nuanced and fascinating, involving a subtle interplay of protons, neutrons, and isotopes. This article will delve deep into the intricacies of atomic mass, explaining what it truly equals and its significance in various scientific fields.
What Atomic Mass Doesn't Equal
Before clarifying what atomic mass does equal, let's dispel a common misconception. Atomic mass is not simply equal to the number of protons in an atom. While the number of protons (the atomic number) defines an element's identity, it only accounts for a portion of the atom's overall mass. Neutrons, which reside in the atom's nucleus alongside protons, also contribute significantly to the atomic mass.
The True Components of Atomic Mass: Protons, Neutrons, and Isotopes
So, what does atomic mass equal? It's the sum of the masses of protons and neutrons in an atom's nucleus. Electrons contribute a negligible amount to the overall mass, so they are usually ignored in atomic mass calculations.
This is where the concept of isotopes becomes vital. Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. This variation in neutron count leads to different atomic masses for isotopes of the same element.
For example, consider carbon. Carbon-12 (¹²C) has 6 protons and 6 neutrons, while carbon-14 (¹⁴C) has 6 protons and 8 neutrons. The superscript number represents the mass number, which is the total number of protons and neutrons. Therefore, carbon-12 has a mass number of 12 and carbon-14 has a mass number of 14. Their atomic masses, while close, are not exactly 12 and 14 amu respectively due to the subtle mass differences between protons and neutrons and the effects of nuclear binding energy.
Atomic Mass Units (amu): Defining the Standard
Atomic mass is typically expressed in atomic mass units (amu), also known as Daltons (Da). One amu is defined as one-twelfth the mass of a single carbon-12 atom. This standard provides a consistent and universally accepted unit for measuring atomic masses.
The choice of carbon-12 as the standard is significant. It's abundant, relatively stable, and its mass can be measured with high precision, facilitating accurate comparisons of atomic masses across various elements.
Average Atomic Mass: Accounting for Isotopic Abundance
Most elements exist in nature as a mixture of isotopes. To account for this natural variation, we use the average atomic mass (also known as average atomic weight), which is a weighted average of the masses of all naturally occurring isotopes of an element. The weighting factor is the relative abundance of each isotope.
For instance, chlorine has two main isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). Chlorine-35 is much more abundant (around 76%) than chlorine-37 (around 24%). Therefore, the average atomic mass of chlorine is not simply the average of 35 and 37, but a weighted average reflecting the abundance of each isotope. The average atomic mass of chlorine is approximately 35.45 amu.
Calculating Average Atomic Mass
The average atomic mass can be calculated using the following formula:
Average Atomic Mass = Σ [(Isotope Mass) x (Isotopic Abundance)]
where:
- Isotope Mass is the mass of a particular isotope in amu.
- Isotopic Abundance is the fractional abundance of that isotope in nature (expressed as a decimal).
- Σ denotes the summation over all isotopes of the element.
Example:
Let's calculate the average atomic mass of an element with two isotopes:
- Isotope 1: Mass = 20 amu, Abundance = 0.70 (70%)
- Isotope 2: Mass = 22 amu, Abundance = 0.30 (30%)
Average Atomic Mass = (20 amu * 0.70) + (22 amu * 0.30) = 14 amu + 6.6 amu = 20.6 amu
The Significance of Atomic Mass in Science and Technology
The atomic mass holds significant importance across various scientific and technological fields:
-
Stoichiometry: In chemistry, atomic mass is crucial for performing stoichiometric calculations, determining the amounts of reactants and products involved in chemical reactions. It enables accurate predictions of reaction yields and the composition of chemical compounds.
-
Nuclear Physics: Atomic mass is essential for understanding nuclear reactions, radioactive decay, and nuclear stability. The mass defect, the difference between the actual mass of a nucleus and the sum of its constituent nucleons’ masses, is a direct consequence of the binding energy that holds the nucleus together.
-
Mass Spectrometry: Mass spectrometry utilizes the mass-to-charge ratio of ions to identify and quantify substances. Atomic mass is a fundamental parameter in this technique, allowing the determination of the elemental composition of samples.
-
Material Science: Understanding atomic mass is critical in developing materials with specific properties. The mass of constituent atoms influences material density, strength, conductivity, and other crucial characteristics.
-
Medical Imaging and Treatment: Techniques like PET (positron emission tomography) and radioisotope therapy rely on the properties of radioactive isotopes, whose atomic masses play a significant role in their applications in diagnosis and treatment of diseases.
-
Geochronology: Atomic masses of isotopes (like uranium-238 and lead-206) are used in radiometric dating techniques to determine the age of rocks and geological formations.
-
Astronomy and Astrophysics: Atomic mass is vital for understanding the composition of stars, planets, and other celestial bodies. Analysis of the spectral lines from stars reveals the relative abundances of elements based on their atomic masses.
Beyond the Basics: Isobaric Analog States and Nuclear Structure
The concept of atomic mass extends beyond simple calculations. It plays a crucial role in understanding more advanced nuclear phenomena, such as isobaric analog states. These are states in different nuclei with the same mass number (A) but different proton and neutron numbers (Z and N). The study of isobaric analog states reveals valuable information about the structure of atomic nuclei and the forces that govern their behavior.
Future Directions and Ongoing Research
Research in atomic mass continues to evolve. Precise measurements of atomic masses are constantly being refined using advanced techniques. This enhanced precision contributes to a deeper understanding of nuclear structure, fundamental interactions, and various applications mentioned earlier. The quest for more accurate atomic mass data fuels ongoing research in nuclear physics and related fields.
Conclusion
In conclusion, the atomic mass is not simply equal to the number of protons, but rather the sum of the masses of protons and neutrons in an atom's nucleus. The concept is inherently linked to isotopes and their relative abundances, resulting in the use of average atomic mass for most elements. Atomic mass is a fundamental concept with widespread applications across numerous scientific disciplines, contributing significantly to our understanding of matter, energy, and the universe around us. From stoichiometric calculations to nuclear physics and astrophysics, the atomic mass remains a cornerstone of scientific progress, continuously driving advancements in our knowledge and technological capabilities.
Latest Posts
Latest Posts
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Atomic Mass Is Equal To The Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
