What Are The Columns In The Periodic Table Called
Muz Play
Mar 19, 2025 · 6 min read
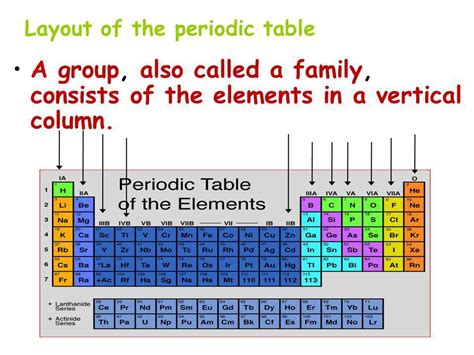
Table of Contents
What Are the Columns in the Periodic Table Called? A Deep Dive into Groups and Families
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While the rows are known as periods, the columns, equally crucial, are referred to as groups or families. Understanding the terminology and the underlying principles behind the organization of groups is essential for comprehending the behavior and interactions of elements. This article delves deep into what these columns represent, exploring their properties and significance in the broader context of chemical understanding.
Groups: The Vertical Organization of the Periodic Table
The columns of the periodic table, the groups, are arranged vertically. Elements within the same group share similar chemical properties because they have the same number of valence electrons. Valence electrons are the electrons in the outermost shell of an atom, which are primarily involved in chemical bonding. This similarity in valence electron configuration directly impacts how atoms of these elements interact with other atoms and molecules.
Understanding Valence Electrons and their Impact on Group Properties
The number of valence electrons dictates an element's reactivity and the types of bonds it can form. For example, elements in Group 1 (alkali metals) have one valence electron, making them highly reactive. They readily lose this electron to form a +1 ion, achieving a stable electron configuration. Conversely, elements in Group 17 (halogens) have seven valence electrons, and they tend to gain one electron to form a -1 ion, achieving a stable octet (eight electrons in the outermost shell). This fundamental principle underpins the predictability and organization of the periodic table.
Group Numbering Systems: A Brief History and Current Conventions
The numbering system for groups has evolved over time. Historically, the groups were numbered using Roman numerals (IA, IIA, IIIB, etc.), reflecting a slightly different grouping system. The current IUPAC (International Union of Pure and Applied Chemistry) system uses Arabic numerals (1, 2, 13-18), which provides a more straightforward and unambiguous method of group identification. Understanding both systems is helpful as older literature might utilize the Roman numeral system.
Exploring Key Groups and Their Characteristic Properties
Let's delve into some prominent groups, highlighting their defining characteristics:
-
Group 1 (Alkali Metals): Highly reactive metals with one valence electron. They readily react with water, producing hydrogen gas and a metal hydroxide. Examples include lithium (Li), sodium (Na), and potassium (K). Their softness and low melting points distinguish them.
-
Group 2 (Alkaline Earth Metals): Also reactive metals, but less so than alkali metals. They have two valence electrons and typically form +2 ions. Examples include magnesium (Mg) and calcium (Ca). They are harder and have higher melting points than alkali metals.
-
Group 13 (Boron Group): This group exhibits a broader range of properties compared to Groups 1 and 2. Elements here have three valence electrons and tend to form +3 ions, although some exhibit other oxidation states. Boron (B) is a metalloid, while aluminum (Al), gallium (Ga), indium (In), and thallium (Tl) are metals.
-
Group 14 (Carbon Group): This group contains elements with four valence electrons. It includes carbon (C), silicon (Si), germanium (Ge), tin (Sn), and lead (Pb). This group encompasses both nonmetals (carbon), metalloids (silicon and germanium), and metals (tin and lead). Carbon, due to its unique ability to form long chains and rings, is the basis of organic chemistry.
-
Group 15 (Pnictogens): Elements in this group have five valence electrons. It includes nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). The properties vary greatly, with nitrogen being a nonmetal, phosphorus a metalloid, and the remaining elements being metals.
-
Group 16 (Chalcogens): This group features elements with six valence electrons, such as oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and polonium (Po). Oxygen is essential for life, while other elements in this group have diverse industrial applications.
-
Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons. They readily gain one electron to form a -1 ion, achieving a stable octet. Fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) are halogens, known for their diverse applications in industry and medicine.
-
Group 18 (Noble Gases): Inert gases with a full outermost electron shell (eight valence electrons, except for helium which has two). This complete electron configuration makes them extremely unreactive, hence their designation as "noble." Helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) are noble gases.
Families: Another Term for Groups
The term "families" is often used synonymously with "groups" to refer to the vertical columns of the periodic table. While "groups" is the more formally accepted scientific term, "families" provides a more intuitive and accessible way to understand the relationships between elements sharing similar chemical properties. The use of "family" emphasizes the close resemblance and shared characteristics within the column.
The Significance of Groups in Chemical Predictions and Applications
The organized arrangement of elements into groups enables chemists to predict the properties of elements and their behavior in chemical reactions. Knowing that an element belongs to a particular group gives insights into:
- Reactivity: The likelihood of an element participating in chemical reactions.
- Oxidation States: The charges an element can adopt when it forms ions or compounds.
- Bonding Behavior: The types of chemical bonds (ionic, covalent, metallic) an element is likely to form.
- Physical Properties: General trends in melting points, boiling points, density, and other physical characteristics can be predicted based on group membership.
This predictive power is invaluable for designing experiments, synthesizing new compounds, and understanding natural phenomena.
Beyond the Main Groups: Transition Metals, Lanthanides, and Actinides
While the main groups discussed above are crucial, the periodic table also includes other sections:
-
Transition Metals: These elements are found in the middle of the periodic table (groups 3-12). They are characterized by variable oxidation states and the formation of colored compounds. Their properties are less predictable based solely on their group number compared to the main group elements.
-
Lanthanides and Actinides: Located at the bottom of the table, these elements represent the f-block elements. They have unique electronic configurations and are characterized by similar chemical properties within their respective series.
Conclusion: Groups – The Key to Understanding the Periodic Table
The columns of the periodic table, known as groups or families, represent a fundamental aspect of chemical organization. The shared number of valence electrons in each group leads to similar chemical properties, enabling predictable behavior and facilitating advancements in chemistry and related fields. Understanding group properties is essential for interpreting chemical reactions, predicting the behavior of elements, and developing new materials and technologies. The periodic table, with its organized arrangement of elements into groups and periods, remains a cornerstone of chemical understanding, providing a framework for understanding the vast diversity of elements and their interactions. By appreciating the systematic organization of the periodic table, particularly the significance of groups, we unlock deeper insights into the world of chemistry.
Latest Posts
Latest Posts
-
Three Structural Components Of An Rna Nucleotide Monomer
Mar 19, 2025
-
The Extraordinary Science Of Addictive Junk Food
Mar 19, 2025
-
What Is The Si Unit For Kinetic Energy
Mar 19, 2025
-
What Is The Difference Between Sex Chromosomes And Autosomes
Mar 19, 2025
-
Vertical Column Of The Periodic Table
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Are The Columns In The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
