Vertical Column Of The Periodic Table
Muz Play
Mar 19, 2025 · 7 min read
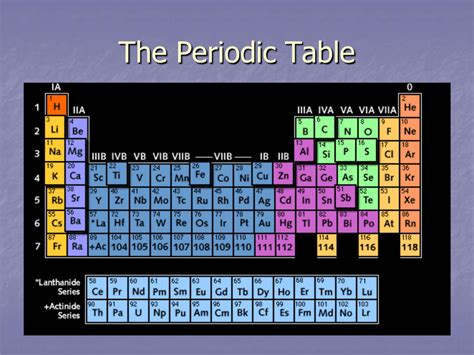
Table of Contents
Delving Deep into the Vertical Columns of the Periodic Table: Groups and Their Properties
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. While the horizontal rows (periods) reveal trends in atomic size and reactivity, the vertical columns, known as groups or families, showcase elements with remarkably similar chemical behaviors. This article explores the fascinating world of these vertical columns, examining their defining characteristics, trends within groups, and the exceptions that enrich our understanding of chemical periodicity.
Understanding Group Properties: A Shared Electron Configuration Story
The defining characteristic of a group is the similar electronic configuration of its constituent elements' outermost electron shell, also known as the valence shell. This similarity directly dictates their chemical reactivity and the types of bonds they form. Elements within a group tend to exhibit similar:
- Oxidation states: This refers to the charge an atom attains when it participates in a chemical bond. Elements in the same group frequently share common oxidation states, although the stability of these states can vary.
- Reactivity: Their shared valence electron configuration heavily influences how readily an element reacts with other substances. For example, alkali metals (Group 1) are highly reactive due to their single valence electron, readily participating in reactions to achieve a stable electron configuration.
- Ionic radii: While the absolute size varies down a group, the relative size changes are consistent, reflecting the addition of electron shells.
- Electronegativity: This property measures an atom's tendency to attract electrons in a chemical bond. Trends in electronegativity are often observed within a group, although the absolute values might vary.
- Physical properties: While not as strictly consistent as chemical properties, elements in the same group often share similar physical states (solid, liquid, gas) under standard conditions and exhibit related melting and boiling points.
Exploring Key Groups of the Periodic Table: A Detailed Look
Let's delve into some specific groups, highlighting their unique characteristics and showcasing the beauty of periodic trends:
Group 1: The Alkali Metals (Li, Na, K, Rb, Cs, Fr)
The alkali metals are renowned for their exceptional reactivity. This high reactivity stems from their single valence electron, which they readily lose to form +1 ions, achieving the stable electron configuration of a noble gas. Key characteristics include:
- Low ionization energies: This means it requires minimal energy to remove their outer electron.
- Low electronegativities: They are not strongly attracted to electrons in chemical bonds.
- Soft and silvery-white metals: They possess a characteristic metallic luster, though they quickly tarnish in air due to their high reactivity.
- React vigorously with water: This reaction produces hydrogen gas and a corresponding alkali metal hydroxide, often with an exothermic (heat-releasing) reaction.
Group 2: The Alkaline Earth Metals (Be, Mg, Ca, Sr, Ba, Ra)
Similar to the alkali metals, alkaline earth metals are reactive metals, though less so than their Group 1 counterparts. Their two valence electrons readily participate in chemical reactions, typically forming +2 ions. Key properties include:
- Higher ionization energies than Group 1: Removing two electrons requires more energy than removing one.
- Higher densities than Group 1: They are generally denser and harder than alkali metals.
- React with water (though less vigorously than Group 1): The reaction produces hydrogen gas and the corresponding metal hydroxide.
- Form stable compounds with oxygen and halogens: Many of their compounds are important in various industrial applications.
Group 17: The Halogens (F, Cl, Br, I, At)
The halogens are highly reactive nonmetals, characterized by their seven valence electrons. They readily gain an electron to form -1 ions, achieving the stable noble gas configuration. This strong electron affinity leads to:
- High electronegativities: They strongly attract electrons in chemical bonds.
- High electron affinities: They readily accept electrons, releasing energy in the process.
- Various oxidation states: Although -1 is the most common oxidation state, other oxidation states are possible for heavier halogens.
- Diverse physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at standard conditions.
Group 18: The Noble Gases (He, Ne, Ar, Kr, Xe, Rn)
The noble gases are unique in their exceptional stability and unreactivity. Their full valence electron shells (eight electrons for most, except helium with two) make them reluctant to participate in chemical reactions. This leads to:
- Very high ionization energies: Removing an electron is extremely difficult.
- Extremely low electronegativities: They have virtually no tendency to attract electrons.
- Colorless, odorless, and monatomic gases: Their inertness is a defining characteristic.
- Limited reactivity: While traditionally considered inert, under specific conditions, some heavier noble gases can form compounds.
Transition Metals (Groups 3-12): A World of Variability
The transition metals, occupying Groups 3-12, represent a diverse group of elements with variable oxidation states and complex chemical behaviors. Their partially filled d orbitals contribute to their unique characteristics:
- Variable oxidation states: Many transition metals can exist in multiple oxidation states, leading to a rich array of compounds.
- Formation of colored compounds: The electronic transitions within the d orbitals often result in colored compounds.
- Catalytic activity: Many transition metals and their compounds act as catalysts, speeding up chemical reactions.
- Magnetic properties: Some transition metals exhibit magnetic properties, such as ferromagnetism (iron, cobalt, nickel).
Post-Transition Metals (Groups 13-16): Bridging the Gap
These elements bridge the gap between the transition metals and the nonmetals. They exhibit properties intermediate between metallic and non-metallic characteristics. Their properties vary significantly depending on their position within the groups.
Lanthanides and Actinides: The Inner Transition Metals
These elements occupy two separate rows below the main body of the periodic table. Their f orbitals contribute to their unique chemical properties. The lanthanides, also known as rare earth elements, are characterized by their similar chemical properties, making their separation and purification challenging. The actinides are all radioactive.
Trends Within Groups: A Systematic Progression
As we move down a group, several trends are typically observed:
- Atomic radius increases: The addition of electron shells leads to a larger atomic size.
- Ionization energy generally decreases: It becomes easier to remove an electron as the atomic radius increases and the outermost electrons are further from the nucleus.
- Electronegativity generally decreases: The attraction for electrons diminishes as the distance from the nucleus increases.
- Metallic character generally increases: Elements become more metallic in their properties as we descend a group.
Exceptions to the Rules: The Intriguing Deviations
While the periodic table provides a framework for understanding element behavior, exceptions exist, adding to the complexity and richness of chemistry. These exceptions arise from various factors, including:
- Electron-electron repulsion: In larger atoms, increased electron-electron repulsion can influence ionization energies and other properties.
- Relativistic effects: At high atomic numbers, relativistic effects on electron velocities can significantly alter properties.
- d-block contraction: The poor shielding effect of d-electrons can lead to a contraction in atomic size.
- f-block contraction: Similar to d-block contraction, the poor shielding of f-electrons leads to a smaller atomic size than expected.
Applications of Group Properties: Real-World Relevance
Understanding the properties of elements within groups has profound practical implications across various fields:
- Materials science: The unique properties of elements within specific groups are crucial in developing novel materials with desired characteristics.
- Medicine: Many elements and their compounds play vital roles in medical applications, from diagnostic tools to therapeutic agents.
- Industry: Industrial processes heavily rely on the chemical reactivity and specific properties of elements from different groups.
- Energy production: The chemical reactions involving elements from different groups are essential in various energy technologies.
- Environmental science: Understanding the environmental impact of elements and their compounds is crucial for sustainability.
Conclusion: The Enduring Significance of Vertical Columns
The vertical columns of the periodic table—the groups—represent a powerful tool for understanding the behavior of elements. Their shared electron configuration dictates their chemical reactivity, physical properties, and their roles in various applications. While trends exist, exceptions and nuances enrich the field of chemistry, making it a fascinating and continuously evolving area of study. Further exploration of these vertical columns promises to unlock new insights into the fundamental building blocks of our world and their myriad applications. The periodic table, with its seemingly simple organization, holds a wealth of information, unlocking the secrets of the universe, one group at a time.
Latest Posts
Latest Posts
-
What Number Uniquely Identifies An Element
Mar 20, 2025
-
An Atom That Has Gained Or Lost Electrons
Mar 20, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Mar 20, 2025
-
How Many Covalent Bonds Does Hydrogen Have
Mar 20, 2025
-
Oxidation Of An Aldehyde Produces A
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Vertical Column Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
