An Atom That Has Gained Or Lost Electrons
Muz Play
Mar 20, 2025 · 7 min read
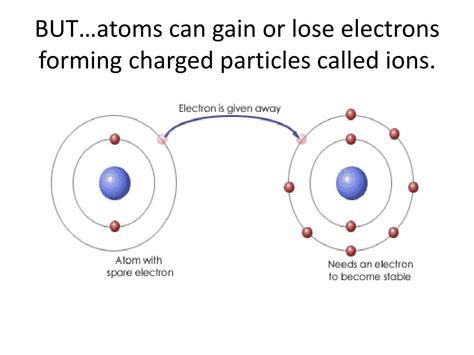
Table of Contents
Ions: When Atoms Gain or Lose Electrons
Atoms, the fundamental building blocks of matter, are typically depicted as neutral entities with a balanced number of protons (positively charged particles) and electrons (negatively charged particles). However, this neutrality is not always the case. Under certain circumstances, atoms can gain or lose electrons, transforming into charged particles known as ions. This seemingly simple process has profound implications across various fields of science and technology, influencing everything from the conductivity of materials to the intricate workings of biological systems. This article delves into the fascinating world of ions, exploring their formation, properties, and significance.
Understanding Ion Formation: The Role of Electronegativity
The driving force behind ion formation is the difference in electronegativity between atoms. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity, like those in Group 17 (halogens), strongly attract electrons, while atoms with low electronegativity, such as those in Group 1 (alkali metals), readily lose electrons.
Cations: Positively Charged Ions
When an atom loses one or more electrons, it becomes positively charged, forming a cation. This loss of electrons occurs because the atom achieves a more stable electronic configuration, often by attaining a full outer electron shell (octet rule). Alkali metals, for example, readily lose one electron to achieve a stable noble gas configuration. Consider sodium (Na): it has one electron in its outermost shell. By losing this electron, it becomes a sodium cation (Na+), possessing a stable configuration similar to neon.
Examples of Cation Formation:
- Sodium (Na) → Na+ + e−: Sodium loses one electron to become a sodium cation.
- Magnesium (Mg) → Mg2+ + 2e−: Magnesium loses two electrons to become a magnesium cation.
- Aluminum (Al) → Al3+ + 3e−: Aluminum loses three electrons to become an aluminum cation.
The ease with which an atom forms a cation is directly related to its ionization energy. Ionization energy is the energy required to remove an electron from a neutral atom. Lower ionization energies indicate a greater tendency to form cations.
Anions: Negatively Charged Ions
Conversely, when an atom gains one or more electrons, it becomes negatively charged, forming an anion. This gain of electrons allows the atom to achieve a more stable electron configuration, typically by completing its outermost shell. Halogens, with seven electrons in their outermost shell, readily gain one electron to achieve a stable noble gas configuration. Chlorine (Cl), for instance, gains one electron to become a chloride anion (Cl−), achieving a stable configuration similar to argon.
Examples of Anion Formation:
- Chlorine (Cl) + e− → Cl−: Chlorine gains one electron to become a chloride anion.
- Oxygen (O) + 2e− → O2−: Oxygen gains two electrons to become an oxide anion.
- Nitrogen (N) + 3e− → N3−: Nitrogen gains three electrons to become a nitride anion.
The ability of an atom to form an anion is related to its electron affinity. Electron affinity is the energy change that occurs when an electron is added to a neutral atom. A higher electron affinity generally indicates a greater tendency to form anions.
Properties of Ions
The properties of ions significantly differ from their neutral atom counterparts. These differences stem from the change in the number of electrons, which directly affects the electrostatic interactions and overall charge distribution within the ion.
Charge and Size
The most fundamental difference lies in their charge. Cations carry a positive charge, while anions carry a negative charge. The magnitude of the charge depends on the number of electrons gained or lost. A singly charged ion (e.g., Na+, Cl−) has a charge of +1 or -1, while a doubly charged ion (e.g., Mg2+, O2−) has a charge of +2 or -2, and so on.
The size of an ion also differs from its parent atom. Cations are generally smaller than their parent atoms, because the loss of electrons reduces electron-electron repulsion, allowing the remaining electrons to be drawn closer to the nucleus. Anions, conversely, are generally larger than their parent atoms, as the addition of electrons increases electron-electron repulsion, causing the electron cloud to expand.
Chemical Reactivity
Ions exhibit significantly different chemical reactivities compared to their neutral atoms. Their charge makes them highly reactive, readily participating in chemical reactions to achieve electrostatic stability. This reactivity is fundamental to the formation of ionic compounds, where cations and anions are held together by strong electrostatic forces of attraction.
Physical Properties
The physical properties of ionic compounds, such as melting point, boiling point, and solubility, are largely determined by the strength of the electrostatic forces between the ions. Ionic compounds typically have high melting and boiling points due to the strong attraction between oppositely charged ions. Their solubility in water depends on the relative strengths of ion-ion interactions and ion-water interactions.
Significance of Ions in Various Fields
The formation and behavior of ions play a critical role in a wide range of scientific and technological fields:
Chemistry
Ions are fundamental to understanding chemical bonding and reactions. Ionic bonding, the electrostatic attraction between cations and anions, is a major type of chemical bond, forming the basis of many important compounds. The properties and reactivity of ionic compounds are directly influenced by the nature of the ions involved. Acid-base reactions, redox reactions, and many other chemical processes fundamentally involve the transfer or sharing of electrons, leading to the formation or interaction of ions.
Biology
Ions are essential for numerous biological processes. Electrolyte balance, the proper concentration of ions like sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) in bodily fluids, is crucial for nerve impulse transmission, muscle contraction, and many other vital functions. Many enzymes require specific ions as cofactors for their catalytic activity. The transport of ions across cell membranes is essential for maintaining cellular homeostasis and facilitating various metabolic processes.
Physics
The movement of ions plays a critical role in various physical phenomena. Ionic conductivity, the ability of a material to conduct electricity through the movement of ions, is an important property of many materials. This property is exploited in various applications, including batteries, fuel cells, and sensors. The study of ion beams and ion implantation is crucial in material science and semiconductor technology.
Geology
Ions are central to understanding geological processes. The formation of minerals often involves the interaction of ions in solution. Weathering, erosion, and the formation of sedimentary rocks all involve the dissolution and precipitation of ionic compounds. The movement of ions in groundwater and other geological fluids influences the composition and properties of rocks and soils.
Medicine
Ions play a vital role in medicine. Electrolyte imbalances can lead to various health problems, requiring medical intervention. Ionic solutions are used in intravenous fluids to replenish electrolytes. The use of ions in medical imaging techniques, such as X-ray and CT scans, helps in diagnosing various diseases.
Technology
Ions have numerous applications in technology. Ionic liquids are increasingly used as solvents in various industrial processes due to their unique properties. Ion-selective electrodes are used to measure the concentration of specific ions in solutions. Ion implantation is used to modify the properties of semiconductor materials.
Conclusion
The formation of ions, through the gain or loss of electrons, is a fundamental process with far-reaching implications. Understanding the factors influencing ion formation, the properties of ions, and their diverse roles in various fields is crucial for advancements in chemistry, biology, physics, geology, medicine, and technology. The study of ions continues to be a vibrant area of research, leading to new discoveries and applications that benefit society as a whole. From the microscopic world of atoms to the macroscopic scale of geological formations and technological innovations, ions are ubiquitous and essential components of the natural and engineered world. Further research into ionic interactions and behavior promises to reveal even more fascinating insights into the fundamental workings of the universe.
Latest Posts
Latest Posts
-
The Elite Theory Of Government Maintains That
Mar 20, 2025
-
What Elements Have An Expanded Octet
Mar 20, 2025
-
Fundamentals Of General Organic And Biological Chemistry
Mar 20, 2025
-
How To Calculate Kb From Ka
Mar 20, 2025
-
Reactions Of Benzene And Substituted Benzenes
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Has Gained Or Lost Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
