What Elements Have An Expanded Octet
Muz Play
Mar 20, 2025 · 6 min read
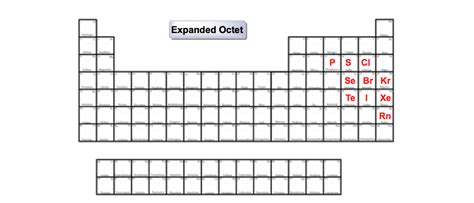
Table of Contents
What Elements Have an Expanded Octet? An In-Depth Look at Hypervalency
The octet rule, a cornerstone of basic chemistry, states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons. This configuration resembles the electron arrangement of noble gases, which are exceptionally unreactive. While a useful guideline for understanding bonding in many molecules, the octet rule isn't universally applicable. Many elements, particularly those in the third period and beyond, can accommodate more than eight electrons in their valence shell, a phenomenon known as hypervalency or having an expanded octet. This article delves into the intricacies of expanded octets, exploring which elements exhibit this behavior, the underlying reasons, and the implications for molecular structure and reactivity.
Understanding the Limitations of the Octet Rule
The octet rule's effectiveness stems from the availability of only s and p orbitals in the valence shell of elements in the second period (Li to Ne). These orbitals can hold a maximum of eight electrons. However, elements in the third period and beyond possess d orbitals in their valence shell. These d orbitals can participate in bonding, allowing for the accommodation of more than eight electrons. This is the key reason why hypervalency is observed primarily in these heavier elements.
Why the Octet Rule Breaks Down
The octet rule is a simplification, a helpful approximation rather than an absolute law. Several factors contribute to its breakdown:
-
Availability of d-orbitals: As mentioned, the presence of d orbitals in the valence shell of third-period elements and beyond is crucial. These orbitals can participate in bonding, accepting additional electrons beyond the octet.
-
Electronegativity Differences: The electronegativity of the central atom and the surrounding atoms plays a significant role. If the surrounding atoms are highly electronegative (like fluorine or oxygen), they can pull electron density away from the central atom, reducing the effective charge and allowing for more electron sharing.
-
Bonding Types: Different types of bonds, such as coordinate covalent bonds (dative bonds) where both electrons originate from the same atom, contribute to expanded octets.
Which Elements Exhibit Expanded Octets?
Elements that commonly display expanded octets are primarily found in the third period and beyond. This includes:
-
Phosphorus (P): Phosphorus pentachloride (PCl₅) and phosphorus pentafluoride (PF₅) are classic examples. In these molecules, phosphorus has ten electrons in its valence shell.
-
Sulfur (S): Sulfur hexafluoride (SF₆) is a well-known example. Sulfur possesses twelve valence electrons in this molecule.
-
Chlorine (Cl): Chlorine can also exhibit expanded octets, although less frequently than phosphorus or sulfur. For example, in compounds like ClF₃ and ClF₅, chlorine exceeds the octet.
-
Silicon (Si): Silicon compounds like SiF₆²⁻ also demonstrate expanded octets.
-
Iodine (I): Iodine forms compounds such as IF₇, which displays a very significantly expanded octet.
It's important to note: While these elements frequently exhibit expanded octets, it's not always the case. The specific molecular environment and the electronegativity of the surrounding atoms influence whether an expanded octet will form.
The Role of d-Orbitals in Hypervalency
The participation of d orbitals in bonding is central to understanding hypervalency. However, the exact mechanism is a subject of ongoing debate among chemists. Two primary models attempt to explain this phenomenon:
1. The d-Orbital Participation Model
This traditional model suggests that d orbitals directly participate in bonding, accepting electrons from surrounding atoms. While intuitively appealing, this model has faced criticism due to the relatively high energy levels of d orbitals in these atoms, making them less readily available for bonding.
2. The Three-Center Four-Electron Bond Model
This alternative model proposes that hypervalent molecules are better described using three-center four-electron (3c-4e) bonds. In this model, three atoms share four electrons, achieving a stable configuration without requiring direct d orbital participation. This model is gaining wider acceptance as it aligns better with computational studies and experimental observations.
Implications for Molecular Structure and Reactivity
The formation of expanded octets has significant implications for molecular geometry and reactivity:
Molecular Geometry
Hypervalent compounds often display unusual molecular geometries. For example:
- PCl₅: Has a trigonal bipyramidal geometry.
- SF₆: Exhibits an octahedral geometry.
- ClF₃: Possesses a T-shaped geometry.
These geometries deviate from those predicted by simple VSEPR (Valence Shell Electron Pair Repulsion) theory, which assumes the octet rule.
Reactivity
Hypervalent compounds can exhibit unique reactivity patterns. For instance:
- SF₆: Is remarkably unreactive due to the strong S-F bonds and the stable octahedral geometry.
- PCl₅: Is a reactive compound, easily hydrolyzed and participating in various substitution reactions.
The reactivity of hypervalent compounds is influenced by factors like bond strengths, steric hindrance, and the nature of the surrounding atoms.
Examples of Compounds with Expanded Octets
Let's delve into specific examples to solidify our understanding:
Phosphorus Pentachloride (PCl₅)
In PCl₅, phosphorus is surrounded by five chlorine atoms. To satisfy the bonding requirements, phosphorus must use its 3s and 3p orbitals, along with potentially 3d orbitals, accommodating more than eight electrons. The molecule adopts a trigonal bipyramidal geometry.
Sulfur Hexafluoride (SF₆)
SF₆ is a remarkably stable and unreactive gas. Sulfur utilizes its 3s and 3p orbitals, and potentially 3d orbitals, to bond with six fluorine atoms, resulting in an expanded octet and an octahedral molecular geometry.
Xenon Compounds
Noble gases, traditionally considered inert, can form compounds under specific conditions. Xenon, in particular, forms compounds like XeF₂ and XeF₄, displaying expanded octets. The high electronegativity of fluorine and the availability of d orbitals in Xenon contribute to this unusual bonding.
Distinguishing Between Hypervalent and Non-Hypervalent Compounds
It's crucial to differentiate between hypervalent and non-hypervalent compounds. While many elements in the third period and beyond can exhibit hypervalency, it's not an inherent property. The molecular environment plays a decisive role. For instance, phosphorus can form both hypervalent (PCl₅) and non-hypervalent (PCl₃) compounds.
Computational Methods and Hypervalency
Modern computational chemistry methods, such as density functional theory (DFT), provide valuable insights into the electronic structure and bonding in hypervalent compounds. These methods help refine our understanding of the extent of d orbital participation and the validity of the 3c-4e bond model.
Conclusion: A Dynamic Area of Chemical Research
The concept of expanded octets and hypervalency remains a dynamic and fascinating area of chemical research. While the octet rule serves as a useful introductory concept, its limitations are evident in the diverse range of compounds exhibiting hypervalency. Understanding the underlying mechanisms and implications of expanded octets is essential for predicting molecular properties and reactivity. Further research continues to refine our understanding of this phenomenon, blending experimental observations with advanced computational techniques. The ongoing debate on the exact nature of bonding in these compounds highlights the complexity and ongoing evolution of our understanding of chemical bonding. The ability of elements to exceed the octet rule significantly expands the possibilities of molecular structures and their diverse chemical behaviors.
Latest Posts
Latest Posts
-
Law Of Segregation Vs Law Of Independent Assortment
Mar 20, 2025
-
Are Substances With A High Melting Point Soluble
Mar 20, 2025
-
Members Of The Kingdom Fungi Are Photosynthetic
Mar 20, 2025
-
Organic Chemistry Substitution And Elimination Reactions Practice Problems
Mar 20, 2025
-
What Is Gas To Solid Called
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Elements Have An Expanded Octet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
