How To Calculate Kb From Ka
Muz Play
Mar 20, 2025 · 5 min read
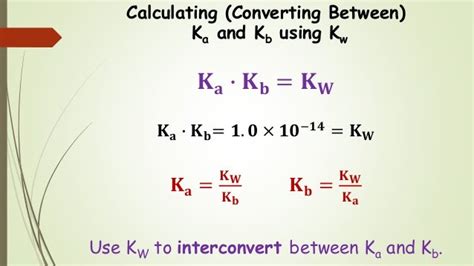
Table of Contents
How to Calculate Kb from Ka: A Comprehensive Guide
Understanding the relationship between Ka and Kb is crucial in acid-base chemistry. This guide provides a comprehensive explanation of how to calculate Kb from Ka, covering the underlying principles, step-by-step calculations, and practical examples. We'll delve into the concepts of acids, bases, dissociation constants, and the ion product of water, equipping you with a solid understanding of this important chemical relationship.
Understanding Ka and Kb: The Dissociation Constants
Before we dive into the calculations, let's establish a firm grasp of Ka and Kb.
Ka, the acid dissociation constant, quantifies the strength of an acid in solution. A higher Ka value indicates a stronger acid, meaning it dissociates more readily into its ions. The general equation for the dissociation of a weak acid, HA, is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The Ka expression is then:
Ka = [H⁺][A⁻] / [HA]
where [H⁺], [A⁻], and [HA] represent the equilibrium concentrations of hydrogen ions, the conjugate base, and the undissociated acid, respectively.
Kb, the base dissociation constant, similarly quantifies the strength of a base in solution. A higher Kb value indicates a stronger base. The general equation for the dissociation of a weak base, B, is:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
The Kb expression is:
Kb = [BH⁺][OH⁻] / [B]
where [BH⁺], [OH⁻], and [B] represent the equilibrium concentrations of the conjugate acid, hydroxide ions, and the undissociated base, respectively.
The Relationship Between Ka and Kb: The Ion Product of Water
The key to calculating Kb from Ka lies in understanding the ion product of water (Kw). Kw represents the equilibrium constant for the autoionization of water:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
At 25°C, Kw has a constant value of 1.0 x 10⁻¹⁴. The expression for Kw is:
Kw = [H₃O⁺][OH⁻] ≈ [H⁺][OH⁻] (Since [H₃O⁺] ≈ [H⁺])
This constant is fundamental because it links the concentrations of hydrogen and hydroxide ions in any aqueous solution.
Calculating Kb from Ka: The Formula and its Derivation
The relationship between Ka and Kb is directly linked to Kw. Consider a conjugate acid-base pair, HA and A⁻. The Ka for HA and the Kb for A⁻ are related through the following equation:
Ka * Kb = Kw
This equation arises from the fact that the dissociation of the acid and the subsequent reaction of its conjugate base with water are linked through the equilibrium constant for water.
Therefore, to calculate Kb from Ka, we simply rearrange this equation:
Kb = Kw / Ka
This formula is universally applicable for conjugate acid-base pairs at a given temperature (usually 25°C, where Kw = 1.0 x 10⁻¹⁴).
Step-by-Step Calculation: A Practical Example
Let's work through a concrete example to solidify our understanding. Suppose we are given the Ka of acetic acid (CH₃COOH) as 1.8 x 10⁻⁵ at 25°C. We want to calculate the Kb of its conjugate base, the acetate ion (CH₃COO⁻).
Step 1: Identify Kw
At 25°C, Kw = 1.0 x 10⁻¹⁴
Step 2: Identify Ka
Ka (acetic acid) = 1.8 x 10⁻⁵
Step 3: Apply the formula
Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰
Therefore, the Kb of the acetate ion is 5.6 x 10⁻¹⁰.
Working with pKa and pKb: A Logarithmic Approach
Often, acid and base strengths are expressed using pKa and pKb, which are the negative logarithms (base 10) of Ka and Kb respectively:
pKa = -log₁₀(Ka) pKb = -log₁₀(Kb)
The relationship between pKa and pKb mirrors the relationship between Ka and Kb:
pKa + pKb = 14 (at 25°C)
This equation simplifies calculations, especially when dealing with very small or very large values of Ka and Kb. To find pKb from pKa, simply subtract the pKa from 14.
Advanced Considerations: Polyprotic Acids and Bases
The calculations become slightly more complex when dealing with polyprotic acids (acids with more than one ionizable proton) or polyprotic bases (bases with more than one ionizable hydroxide group). Each dissociation step will have its own Ka or Kb value. For example, a diprotic acid (like sulfuric acid) will have a Ka1 and a Ka2. The calculation of Kb for the conjugate base will depend on which Ka value is being considered.
The overall strategy remains the same; however, you need to carefully consider which dissociation step you are working with and use the appropriate Ka value in the equation Kb = Kw / Ka.
Practical Applications and Importance of Ka and Kb Calculations
The ability to calculate Kb from Ka (or vice versa) has widespread applications in various fields:
-
Buffer Solutions: Understanding Ka and Kb is essential in designing and analyzing buffer solutions, crucial in maintaining stable pH levels in biological systems and chemical processes.
-
Titration Curves: These calculations help in predicting the shape and equivalence point of titration curves, used in analytical chemistry for determining the concentration of unknown solutions.
-
Solubility Equilibria: The concept extends to understanding the solubility of slightly soluble salts, where the solubility product constant (Ksp) plays a similar role.
-
Environmental Chemistry: Calculations involving Ka and Kb are vital in understanding acid rain, water purification processes, and the effects of pollutants on aquatic environments.
-
Biochemistry and Medicine: Many biochemical processes involve acids and bases, and understanding their dissociation constants is crucial for understanding enzyme activity, drug efficacy, and metabolic pathways.
Conclusion: Mastering the Ka-Kb Relationship
Calculating Kb from Ka is a fundamental skill in acid-base chemistry. This guide has provided a comprehensive walkthrough, covering the underlying theory, step-by-step calculations, practical examples, and advanced considerations. By mastering this relationship, you gain a deeper understanding of acid-base equilibria and its significance across various scientific disciplines. Remember the core relationship: Ka * Kb = Kw, and you'll be well-equipped to tackle these calculations with confidence. This understanding forms the bedrock for further explorations into more complex aspects of acid-base chemistry. Practice applying these principles and continue to explore the rich world of chemical equilibria!
Latest Posts
Latest Posts
-
Are Substances With A High Melting Point Soluble
Mar 20, 2025
-
Members Of The Kingdom Fungi Are Photosynthetic
Mar 20, 2025
-
Organic Chemistry Substitution And Elimination Reactions Practice Problems
Mar 20, 2025
-
What Is Gas To Solid Called
Mar 20, 2025
-
Moment Of Inertia Of Rectangular Prism
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Kb From Ka . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
