Oxidation Of An Aldehyde Produces A
Muz Play
Mar 20, 2025 · 6 min read
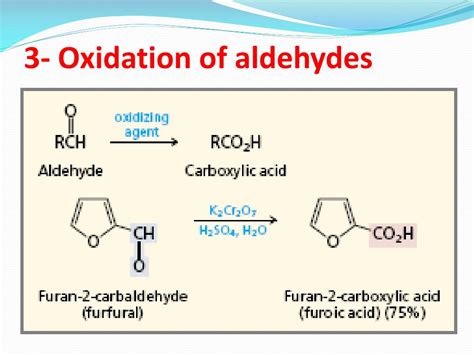
Table of Contents
The Oxidation of an Aldehyde: Unveiling the Products and Mechanisms
The oxidation of aldehydes, a fundamental reaction in organic chemistry, holds significant importance in various industrial processes and biological pathways. Understanding the products formed and the mechanisms driving this transformation is crucial for chemists, biochemists, and anyone working with organic compounds. This comprehensive article delves into the intricacies of aldehyde oxidation, exploring the various reaction pathways, influencing factors, and the diverse applications of the resulting products.
Understanding Aldehydes: Structure and Reactivity
Before diving into the oxidation process, let's briefly review the structure and reactivity of aldehydes. Aldehydes are characterized by a carbonyl group (C=O) bonded to at least one hydrogen atom. This structural feature dictates their unique chemical behavior, making them susceptible to oxidation. The presence of the polar carbonyl group, with its electron-withdrawing oxygen atom, renders the α-carbon (carbon adjacent to the carbonyl) slightly electrophilic, making it vulnerable to nucleophilic attacks. Furthermore, the hydrogen atom bonded to the carbonyl carbon is easily oxidized due to its relatively low bond dissociation energy.
Oxidation Pathways: Unveiling the Products
The oxidation of an aldehyde typically yields a carboxylic acid. This is the most common and straightforward outcome. The reaction involves the breaking of the C-H bond on the aldehyde's carbonyl group and the formation of a new C-O bond, ultimately transforming the aldehyde's carbonyl group into a carboxylic acid group (-COOH).
Several oxidizing agents can facilitate this transformation, each with its own mechanism and selectivity. Some common oxidizing agents include:
-
Potassium permanganate (KMnO₄): A strong oxidizing agent that can oxidize aldehydes to carboxylic acids even under mild conditions. It's often used in aqueous solutions.
-
Chromic acid (H₂CrO₄): Another potent oxidant, typically used in the form of chromic anhydride (CrO₃) or potassium dichromate (K₂Cr₂O₇) in acidic solutions. It's known for its effectiveness but is less environmentally friendly due to the toxicity of chromium.
-
Tollen's reagent ([Ag(NH₃)₂]⁺): A mild oxidizing agent that's particularly useful for distinguishing between aldehydes and ketones. The aldehyde is oxidized to a carboxylic acid, while the silver ions in the reagent are reduced to metallic silver, forming a characteristic silver mirror.
-
Benedict's reagent and Fehling's solution: These are copper-based reagents that also selectively oxidize aldehydes, reducing the copper ions to a reddish-brown precipitate of copper(I) oxide. These reagents are often used in qualitative tests for aldehydes.
-
Jones reagent (CrO₃ in aqueous sulfuric acid): A powerful oxidizing agent capable of oxidizing primary and secondary alcohols, and, of course, aldehydes to carboxylic acids. However, the use of Jones reagent requires careful control of reaction conditions.
Mechanisms of Aldehyde Oxidation
The precise mechanism of aldehyde oxidation varies slightly depending on the oxidizing agent used. However, most mechanisms share some common features. The general steps involved often include:
-
Nucleophilic attack: The oxidizing agent, or a species derived from it, acts as an electrophile and attacks the carbonyl carbon. This can involve a hydride abstraction or the formation of an intermediate complex.
-
Hydride transfer: A crucial step in many aldehyde oxidation mechanisms is the transfer of a hydride ion (H⁻) from the aldehyde to the oxidizing agent. This step often involves the breaking of the C-H bond on the aldehyde's carbonyl carbon.
-
Formation of a carboxylic acid derivative: Following hydride transfer, the intermediate species reorganizes to form a derivative of the carboxylic acid. This can be the carboxylic acid itself or a related species that is readily hydrolyzed to the carboxylic acid.
-
Workup: The final step involves workup procedures to isolate the carboxylic acid product. This often entails quenching the reaction mixture with water or an aqueous solution and subsequent extraction or crystallization.
For example, in the oxidation of an aldehyde with chromic acid, the mechanism involves the formation of a chromate ester intermediate, followed by hydride transfer and the subsequent decomposition of the intermediate to yield the carboxylic acid and chromium(III) ions. Tollen's reagent oxidation involves a direct hydride transfer to the silver ion, reducing it to metallic silver and forming a carboxylate ion that is later protonated to give the carboxylic acid.
Factors Affecting Aldehyde Oxidation
Several factors can influence the rate and efficiency of aldehyde oxidation:
-
The nature of the oxidizing agent: The strength and reactivity of the oxidizing agent significantly impacts the reaction rate. Stronger oxidizing agents generally lead to faster reactions.
-
Solvent effects: The solvent used can affect the solubility of both the aldehyde and the oxidizing agent, influencing the reaction rate and selectivity. Polar solvents often favor oxidation reactions.
-
pH: The pH of the reaction mixture can significantly affect the reaction rate and selectivity. Acidic conditions are frequently favored for many oxidizing agents.
-
Temperature: Higher temperatures generally accelerate the reaction rate, but excessive heat can lead to side reactions or decomposition.
Applications of Carboxylic Acids from Aldehyde Oxidation
The carboxylic acids produced by the oxidation of aldehydes have a vast array of applications across various fields:
-
Industrial production: Carboxylic acids are vital building blocks in the chemical industry. They are used as precursors in the synthesis of numerous chemicals, including esters, amides, and other functional groups. For example, acetic acid (from acetaldehyde oxidation) is widely used in the production of plastics, solvents, and other industrial chemicals.
-
Pharmaceuticals: Many carboxylic acids and their derivatives are crucial components of pharmaceuticals. They might act as active ingredients or serve as intermediates in drug synthesis.
-
Food and beverage industry: Certain carboxylic acids, like citric acid, are widely used as food additives, flavoring agents, and preservatives.
-
Cosmetics and personal care products: Carboxylic acids and their derivatives are present in many cosmetic products, serving as emulsifiers, preservatives, and pH adjusters.
Beyond Carboxylic Acids: Other Oxidation Products (Under Specific Conditions)
While the formation of a carboxylic acid is the most prevalent outcome of aldehyde oxidation, under specific conditions, other products may arise. For instance:
-
Over-oxidation: With particularly strong oxidizing agents and harsh conditions, further oxidation of the carboxylic acid can occur, leading to the formation of carbon dioxide and water. This complete oxidation is less common with carefully controlled reactions.
-
Baeyer-Villiger Oxidation: This reaction, using a peroxyacid as the oxidant, transforms the aldehyde into an ester. This pathway is distinct from direct carboxylic acid formation and requires a specific type of oxidizing agent.
Conclusion
The oxidation of aldehydes, predominantly yielding carboxylic acids, is a significant reaction with far-reaching implications across various disciplines. Understanding the mechanisms, influencing factors, and diverse applications of the resulting products is crucial for anyone working with organic compounds. This reaction plays a pivotal role in industrial processes, biological pathways, and the synthesis of numerous commercially important chemicals. Further research continues to refine our understanding of the intricacies of aldehyde oxidation, paving the way for new applications and synthetic strategies. The diverse nature of oxidizing agents allows for fine-tuning the reaction, providing chemists with powerful tools for achieving specific synthetic goals. The future holds exciting possibilities for the further exploitation of aldehyde oxidation reactions in the development of new materials and technologies.
Latest Posts
Latest Posts
-
Bundles Of Axons Within The Central Nervous System Are Called
Mar 20, 2025
-
What Is The Basic Unit Of Heredity
Mar 20, 2025
-
Why Is Equatorial More Stable Than Axial
Mar 20, 2025
-
Chemistry A Molecular Approach Nivaldo Tro
Mar 20, 2025
-
Humidity Is Measured With What Instrument
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Of An Aldehyde Produces A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
