Compounds Containing Only Carbon And Hydrogen Are Called
Muz Play
Mar 20, 2025 · 6 min read
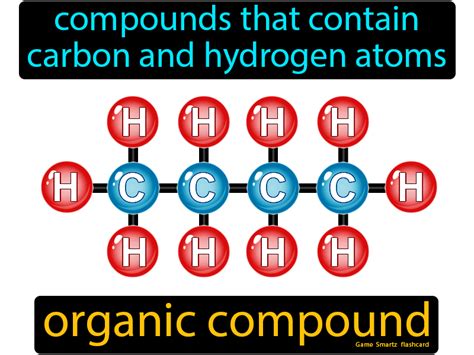
Table of Contents
Compounds Containing Only Carbon and Hydrogen Are Called Hydrocarbons: A Comprehensive Guide
Compounds containing only carbon and hydrogen are called hydrocarbons. These fundamental organic molecules form the basis of countless other organic compounds and are crucial to various industries, from energy production to plastics manufacturing. Understanding their structure, properties, and classification is key to grasping the vast world of organic chemistry. This comprehensive guide delves deep into the fascinating realm of hydrocarbons, exploring their different types, characteristics, and applications.
Understanding the Building Blocks: Carbon and Hydrogen
Before diving into the intricacies of hydrocarbons, it's essential to understand the unique properties of carbon and hydrogen that allow them to form such a diverse array of molecules.
Carbon's Versatility:
Carbon, with its four valence electrons, possesses an unparalleled ability to form strong covalent bonds with itself and other elements. This characteristic enables carbon to create long chains, branched structures, and rings, leading to the vast structural diversity observed in hydrocarbons and organic compounds in general. This ability to catenate, or bond to itself, is a key factor in the existence of millions of different organic compounds.
Hydrogen's Simplicity:
Hydrogen, with its single valence electron, readily forms stable covalent bonds with carbon. Its relative simplicity contributes to the predictable nature of many hydrocarbon reactions. The C-H bond is relatively strong and non-polar, influencing the physical and chemical properties of hydrocarbons.
The Diverse World of Hydrocarbons: Classification and Properties
Hydrocarbons are classified into two main categories based on the type of bonds present between carbon atoms:
1. Aliphatic Hydrocarbons: The Linear and Branched Family
Aliphatic hydrocarbons feature carbon atoms linked in open chains, either straight or branched. They are further subdivided into:
a) Alkanes: The Saturated Heroes
Alkanes are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. Their general formula is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. Alkanes are relatively unreactive compared to other hydrocarbons due to the strength and stability of their single bonds.
- Examples: Methane (CH₄), Ethane (C₂H₆), Propane (C₃H₈), Butane (C₄H₁₀)
- Properties: Generally non-polar, insoluble in water, less dense than water, relatively low boiling points (increasing with molecular weight). They are primarily used as fuels.
b) Alkenes: The Double-Bonded Dynamic Duo
Alkenes are unsaturated hydrocarbons containing at least one carbon-carbon double bond (C=C). Their general formula is C<sub>n</sub>H<sub>2n</sub>. The presence of the double bond introduces a region of higher electron density, making alkenes more reactive than alkanes. They readily undergo addition reactions.
- Examples: Ethene (C₂H₄), Propene (C₃H₆), Butene (C₄H₈)
- Properties: Similar to alkanes in terms of polarity and density, but generally have higher boiling points due to stronger intermolecular forces. They serve as important building blocks in the production of polymers (plastics).
c) Alkynes: The Triple-Threat
Alkynes are also unsaturated hydrocarbons, featuring at least one carbon-carbon triple bond (C≡C). Their general formula is C<sub>n</sub>H<sub>2n-2</sub>. The presence of the triple bond makes alkynes the most reactive of the aliphatic hydrocarbons, readily participating in addition reactions.
- Examples: Ethyne (C₂H₂, commonly known as acetylene), Propyne (C₃H₄)
- Properties: Similar to alkenes, but generally even higher boiling points due to stronger intermolecular forces. Acetylene is used in welding and cutting due to its high heat of combustion.
2. Aromatic Hydrocarbons: The Cyclic Champions
Aromatic hydrocarbons, also known as arenes, are characterized by the presence of a benzene ring, a six-carbon ring with alternating single and double bonds. This unique structure results in a delocalized electron cloud above and below the plane of the ring, imparting special stability and reactivity.
a) Benzene and its Derivatives:
Benzene (C₆H₆) is the simplest aromatic hydrocarbon. Its unique structure, often represented by a hexagon with a circle inside, indicates the delocalized electrons. Many other aromatic compounds are derivatives of benzene, with various substituents attached to the ring.
- Examples: Toluene (methylbenzene), Phenol (hydroxybenzene)
- Properties: Relatively non-polar, less dense than water, and have relatively high boiling points compared to aliphatic hydrocarbons of similar molecular weight. They are used extensively in various industrial applications, including the production of plastics, dyes, and pharmaceuticals.
Isomerism: The Same Formula, Different Structures
A significant aspect of hydrocarbon chemistry is isomerism. Isomers are molecules with the same molecular formula but different structural arrangements. This leads to variations in their physical and chemical properties. Isomerism is particularly prevalent in hydrocarbons with four or more carbon atoms.
- Structural Isomers: These differ in the way atoms are connected. For example, butane (C₄H₁₀) has two structural isomers: n-butane (a straight chain) and isobutane (a branched chain).
- Geometric Isomers (cis-trans isomers): These arise in alkenes due to the restricted rotation around the carbon-carbon double bond. The substituents on the double bond can be arranged on the same side (cis) or opposite sides (trans), leading to different properties.
Nomenclature: Naming the Hydrocarbons
A systematic naming system, based on IUPAC (International Union of Pure and Applied Chemistry) rules, is used to name hydrocarbons. The names indicate the number of carbon atoms, the type of bonds, and the presence of any branches or functional groups.
Understanding the nomenclature is crucial for identifying and classifying different hydrocarbons. The basic principles involve identifying the longest carbon chain, numbering the carbons, naming the substituents, and assigning the appropriate prefix and suffix.
Applications of Hydrocarbons: Fueling Progress
Hydrocarbons play a pivotal role in modern society, serving as the backbone of many industries. Their applications are vast and diverse:
- Fuels: Alkanes, particularly methane, propane, and butane, are essential components of natural gas and petroleum. They are widely used as fuels for heating, transportation, and electricity generation.
- Plastics and Polymers: Alkenes are the building blocks for the production of a wide range of plastics and polymers, essential materials used in packaging, construction, and various other applications.
- Solvents: Certain hydrocarbons are used as solvents in various industrial processes, such as cleaning, extraction, and dissolving substances.
- Lubricants: Some hydrocarbons serve as lubricants in engines and machinery, reducing friction and wear.
- Pharmaceuticals and other chemicals: Aromatic hydrocarbons and their derivatives are crucial intermediates in the synthesis of numerous pharmaceuticals, dyes, and other chemicals.
Environmental Concerns: Balancing Progress with Sustainability
While hydrocarbons are essential for modern life, their extraction, processing, and combustion raise environmental concerns. The burning of fossil fuels (containing hydrocarbons) releases greenhouse gases, contributing to climate change. Furthermore, oil spills and other forms of pollution associated with hydrocarbon extraction and transportation can have devastating consequences for the environment. Therefore, exploring alternative energy sources and developing more sustainable methods for hydrocarbon utilization is crucial for ensuring a healthier planet.
Conclusion: A Foundation for Organic Chemistry
Hydrocarbons form the cornerstone of organic chemistry. Understanding their structure, properties, and classifications is essential for comprehending the vast array of organic compounds and their applications. Their versatility and importance are undeniable, but it's crucial to address the environmental challenges associated with their use to ensure a sustainable future. The continued research and development in this field will undoubtedly lead to new discoveries and applications, furthering our understanding of this fundamental class of organic molecules. Further exploration into specific hydrocarbon reactions, like combustion, addition reactions, and substitution reactions, can provide a more complete picture of their chemical behavior. The ongoing study of hydrocarbons, their derivatives, and their environmental impact will continue to shape our world for years to come.
Latest Posts
Latest Posts
-
Kirby Bauer Method Of Antibiotic Sensitivity Testing
Mar 20, 2025
-
Are Covalent Bonds Soluble In Water
Mar 20, 2025
-
What Are The Products Of The Neutralization Reaction
Mar 20, 2025
-
Where Does Oxidation Occur In A Voltaic Cell
Mar 20, 2025
-
The Cranial Cavity Is A Subdivision Of The
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Compounds Containing Only Carbon And Hydrogen Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
