What Are The Products Of The Neutralization Reaction
Muz Play
Mar 20, 2025 · 6 min read
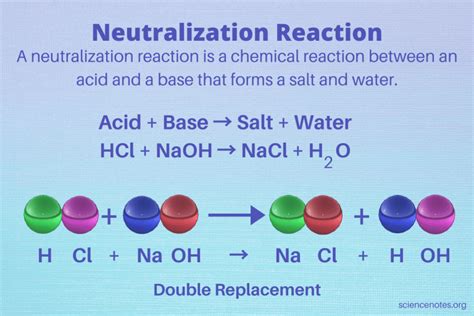
Table of Contents
What Are the Products of a Neutralization Reaction? A Deep Dive into Acid-Base Chemistry
Neutralization reactions are fundamental chemical processes that occur when an acid and a base react quantitatively with each other. Understanding the products of these reactions is crucial for various applications, from everyday life to advanced chemical industries. This comprehensive guide will explore the various products formed in neutralization reactions, delving into the specifics of different acid-base combinations and highlighting their practical significance.
The Fundamentals: Acids, Bases, and Neutralization
Before diving into the products, let's revisit the basics. Acids are substances that donate protons (H⁺ ions) when dissolved in water, increasing the concentration of H⁺ ions. Bases, on the other hand, accept protons or release hydroxide ions (OH⁻ ions) in water, increasing the concentration of OH⁻ ions.
A neutralization reaction is essentially a double displacement reaction where the hydrogen ions from the acid and the hydroxide ions from the base combine to form water (H₂O). This is the most common and characteristic product of a neutralization reaction. The remaining ions from the acid and the base combine to form a salt.
Therefore, the general equation for a neutralization reaction is:
Acid + Base → Salt + Water
Common Types of Neutralization Reactions and Their Products
The specific salt formed depends entirely on the acid and base used in the reaction. Let's explore some common examples:
1. Strong Acid - Strong Base Neutralization
This type of neutralization reaction is characterized by the complete dissociation of both the acid and the base in water. The reaction proceeds to completion, and the resulting solution is neutral (pH 7) if stoichiometrically equivalent amounts of acid and base are used.
Example: The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
Here, the products are sodium chloride (NaCl), a common salt, and water (H₂O). The solution is neutral due to the complete neutralization of H⁺ and OH⁻ ions.
2. Strong Acid - Weak Base Neutralization
In this case, a strong acid completely dissociates, while the weak base only partially dissociates. The resulting solution will be slightly acidic (pH < 7) because the conjugate acid of the weak base remains in solution.
Example: The reaction between hydrochloric acid (HCl) and ammonia (NH₃):
HCl (aq) + NH₃ (aq) → NH₄Cl (aq)
The product is ammonium chloride (NH₄Cl), a salt, and water is implicitly formed as H⁺ from HCl reacts with NH₃ to produce NH₄⁺. The solution is slightly acidic due to the presence of the ammonium ion (NH₄⁺), which acts as a weak acid.
3. Weak Acid - Strong Base Neutralization
Similar to the previous case, but in reverse. The weak acid partially dissociates, while the strong base completely dissociates. The resulting solution will be slightly basic (pH > 7) because the conjugate base of the weak acid remains in solution.
Example: The reaction between acetic acid (CH₃COOH) and sodium hydroxide (NaOH):
CH₃COOH (aq) + NaOH (aq) → CH₃COONa (aq) + H₂O (l)
The products are sodium acetate (CH₃COONa), a salt, and water (H₂O). The solution is slightly basic due to the presence of the acetate ion (CH₃COO⁻), which acts as a weak base.
4. Weak Acid - Weak Base Neutralization
Both the acid and the base partially dissociate. Predicting the pH of the resulting solution is more complex and depends on the relative strengths of the acid and base. The solution might be acidic, basic, or neutral depending on the pKa and pKb values of the acid and base.
Example: The reaction between acetic acid (CH₃COOH) and ammonia (NH₃):
CH₃COOH (aq) + NH₃ (aq) ⇌ CH₃COONH₄ (aq)
The product is ammonium acetate (CH₃COONH₄), a salt. The pH of the solution is determined by the relative strengths of the acetate and ammonium ions as weak acid and weak base. This requires careful consideration of the equilibrium constants.
Properties and Uses of Salt Products
The salt formed in a neutralization reaction is a crucial product with diverse applications. The properties of the salt depend entirely on the constituent ions. Salts can be:
- Neutral: Salts formed from strong acid-strong base reactions are generally neutral in solution. Example: NaCl.
- Acidic: Salts formed from a strong acid and a weak base are usually acidic. Example: NH₄Cl.
- Basic: Salts formed from a weak acid and a strong base are generally basic. Example: CH₃COONa.
These properties have important implications in various applications:
- Food industry: Many salts are used as flavor enhancers, preservatives, and acidity regulators. Sodium chloride (NaCl) is the most common example.
- Medicine: Certain salts are used as electrolytes to maintain fluid balance in the body. Others have therapeutic applications.
- Agriculture: Salts are used as fertilizers to provide essential nutrients to plants.
- Industry: Various salts are used in manufacturing processes, such as in the production of detergents, plastics, and dyes.
Beyond the Basics: Considerations and Exceptions
While the simple equation "Acid + Base → Salt + Water" provides a general understanding, some nuances require further consideration:
-
Gas evolution: Some neutralization reactions involving certain acids and bases may result in the evolution of a gas, such as carbon dioxide (CO₂) from the reaction of a carbonate or bicarbonate with an acid. For example:
2HCl (aq) + CaCO₃ (s) → CaCl₂ (aq) + H₂O (l) + CO₂ (g)
-
Precipitation reactions: If the salt formed is insoluble in water, it will precipitate out of the solution as a solid. This is an example of a neutralization reaction that also involves a precipitation reaction. For example:
2NaOH (aq) + CuSO₄ (aq) → Na₂SO₄ (aq) + Cu(OH)₂ (s)
-
Heat of neutralization: Neutralization reactions are generally exothermic, meaning they release heat. The amount of heat released can vary depending on the strength of the acid and base involved.
Applications and Importance of Neutralization Reactions
Neutralization reactions have widespread applications across various fields:
- Wastewater treatment: Neutralization is used to adjust the pH of wastewater before discharge, minimizing environmental impact.
- Chemical synthesis: Neutralization reactions are employed in the synthesis of many organic and inorganic compounds.
- Titration: Acid-base titrations utilize neutralization reactions to determine the concentration of an unknown acid or base.
- Medicine: Antacids work by neutralizing excess stomach acid.
- Agriculture: Soil pH adjustment often involves neutralization reactions to create optimal conditions for plant growth.
Conclusion
Neutralization reactions are fundamental chemical processes with far-reaching consequences. Understanding the products of these reactions – primarily salts and water – and the properties of those salts, is crucial for a wide range of applications. The complexity can vary depending on the strength of the acid and base involved, with possibilities of gas evolution and precipitation reactions further enriching the landscape of these essential chemical interactions. By mastering the fundamentals and exploring the nuances, we unlock a deeper appreciation for the power and versatility of neutralization reactions in various scientific and technological domains.
Latest Posts
Latest Posts
-
Calculating Entropy Change From Reversible Heat Flow
Mar 20, 2025
-
The Building Blocks Of Carbohydrates Are
Mar 20, 2025
-
Chemiosmosis Atp Synthesis In Chloroplasts Answer Key
Mar 20, 2025
-
How To Find Expected Frequency From Observed Frequency
Mar 20, 2025
-
Confidence Interval For Population Means Calculator
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Are The Products Of The Neutralization Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
