Are Covalent Bonds Soluble In Water
Muz Play
Mar 20, 2025 · 6 min read
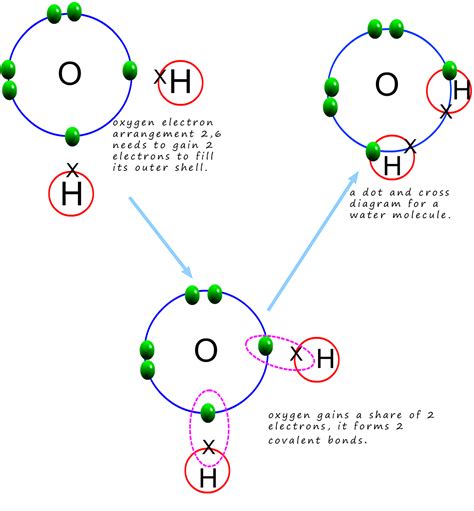
Table of Contents
Are Covalent Bonds Soluble in Water? A Deep Dive into Polarity, Hydrogen Bonding, and Solubility
The question of whether covalent bonds are soluble in water isn't a simple yes or no. The solubility of a covalent compound in water is far more nuanced and depends heavily on the polarity of the molecule. While the presence of covalent bonds itself doesn't dictate solubility, the nature of the bonds and the resulting molecular structure play a crucial role. Let's delve into the intricacies of this fascinating topic.
Understanding Covalent Bonds and Water
Before exploring solubility, let's refresh our understanding of covalent bonds and the unique properties of water.
Covalent Bonds: Sharing is Caring
A covalent bond forms when two atoms share electrons to achieve a more stable electron configuration. This sharing creates a relatively strong bond, holding the atoms together in a molecule. Covalent bonds are prevalent in organic molecules, forming the backbone of proteins, carbohydrates, lipids, and nucleic acids. Examples include the bonds in methane (CH₄), glucose (C₆H₁₂O₆), and ethanol (C₂H₅OH).
Water: The Universal Solvent (But Not for Everything!)
Water (H₂O) is a remarkable solvent, known as the "universal solvent" due to its ability to dissolve a wide range of substances. This exceptional solvating power stems from its polar nature and its capacity to form hydrogen bonds.
-
Polarity: The oxygen atom in water is more electronegative than the hydrogen atoms, meaning it attracts electrons more strongly. This creates a polar molecule with a slightly negative charge (δ-) near the oxygen and slightly positive charges (δ+) near the hydrogens.
-
Hydrogen Bonding: The strong electronegativity of oxygen coupled with the small size of hydrogen atoms allows water molecules to form hydrogen bonds with each other and with other polar molecules. Hydrogen bonds are relatively weak compared to covalent bonds, but their collective effect is significant in determining the properties of water and its ability to dissolve certain substances.
The Key to Solubility: "Like Dissolves Like"
The principle of "like dissolves like" is central to understanding solubility. Polar solvents, such as water, tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. This is because:
-
Polar-Polar Interactions: Polar molecules interact favorably with each other through dipole-dipole interactions and hydrogen bonding. The positive end of one polar molecule attracts the negative end of another, leading to solvation.
-
Nonpolar-Nonpolar Interactions: Nonpolar molecules interact through weak London dispersion forces. These forces are relatively weak, but they become significant in large nonpolar molecules. Nonpolar solvents can effectively break down these weak forces and surround nonpolar solutes, leading to dissolution.
-
Polar-Nonpolar Interactions: Polar and nonpolar molecules have limited interaction. The strong attractive forces between polar molecules in water make it difficult for water to effectively surround and dissolve nonpolar solutes. This results in poor or negligible solubility.
When Covalent Compounds Dissolve in Water: A Detailed Look
Now, let's explore specific cases where covalent compounds exhibit different levels of solubility in water.
1. Polar Covalent Compounds: High Solubility
Many polar covalent compounds readily dissolve in water. Their polarity allows them to interact favorably with water molecules through dipole-dipole interactions and, in some cases, hydrogen bonding.
Examples:
- Sugars (e.g., glucose): Glucose contains multiple hydroxyl (-OH) groups, which are highly polar and capable of forming hydrogen bonds with water molecules, leading to high solubility.
- Alcohols (e.g., ethanol): Alcohols contain a hydroxyl (-OH) group, contributing to their polarity and solubility in water. Smaller alcohols are more soluble than larger ones because the hydrophobic (water-repelling) alkyl chain becomes increasingly dominant as the size increases.
- Carboxylic acids (e.g., acetic acid): The carboxyl group (-COOH) in carboxylic acids is highly polar and capable of forming hydrogen bonds with water.
- Amines (e.g., methylamine): Amines contain an amino group (-NH₂) that can form hydrogen bonds with water.
Factors Influencing Solubility:
- Number and type of polar functional groups: More polar groups generally lead to higher solubility.
- Size and shape of the molecule: Larger molecules or molecules with complex shapes may hinder their interaction with water, reducing solubility even if they possess polar groups.
2. Nonpolar Covalent Compounds: Low Solubility
Nonpolar covalent compounds generally exhibit low solubility in water. Their lack of polarity prevents strong interactions with water molecules, resulting in poor dissolution.
Examples:
- Lipids (e.g., fats and oils): Fats and oils are composed primarily of long hydrocarbon chains, which are nonpolar and hydrophobic.
- Aromatic hydrocarbons (e.g., benzene): These compounds have delocalized electrons that do not create significant polarity.
- Alkanes (e.g., methane, ethane): Alkanes are completely nonpolar and are almost insoluble in water.
Factors Influencing Solubility (the lack of it):
- Length of hydrocarbon chains: Longer chains lead to lower solubility.
- Branching: Branching can slightly increase solubility compared to linear chains of the same length, but the effect is minor.
3. Amphipathic Covalent Compounds: Unique Behavior
Amphipathic molecules possess both polar and nonpolar regions. Their behavior in water is unique.
Examples:
- Fatty acids: Fatty acids have a polar carboxyl head and a long nonpolar hydrocarbon tail.
- Phospholipids: Phospholipids are crucial components of cell membranes. They have polar phosphate heads and nonpolar fatty acid tails.
- Soaps and detergents: These are surfactants that contain both polar and nonpolar parts, allowing them to interact with both water and grease.
In water, amphipathic molecules tend to form micelles or bilayers. The polar heads interact with water, while the nonpolar tails cluster together, minimizing their contact with water. This behavior is crucial for the formation of cell membranes and the action of soaps and detergents.
Factors Affecting Solubility Beyond Polarity
While polarity is the primary determinant of solubility, other factors can also influence whether a covalent compound dissolves in water:
- Temperature: Generally, increasing the temperature increases the solubility of solids and liquids in water.
- Pressure: Pressure primarily affects the solubility of gases in water; increased pressure generally increases solubility.
- Hydrogen bonding: The ability of a molecule to form hydrogen bonds with water significantly enhances its solubility.
- Steric hindrance: Bulky groups around polar functional groups can hinder interactions with water, decreasing solubility.
- Molecular weight: Higher molecular weight often correlates with lower solubility, particularly in nonpolar molecules.
Conclusion: It's All About the Interactions
The solubility of a covalent compound in water isn't simply about the presence of covalent bonds; it's about the overall polarity of the molecule and its ability to interact with water molecules through various forces. Polar covalent compounds with many polar functional groups generally exhibit high solubility, while nonpolar compounds show low solubility. Amphipathic molecules display unique behavior, forming structures that minimize contact between their nonpolar parts and water. By understanding these principles, we can predict and explain the solubility of a wide variety of covalent compounds in water, a crucial concept in chemistry and various related fields.
Latest Posts
Latest Posts
-
Elements Of Group 17 Are Called
Mar 20, 2025
-
Is A Polymer Of Amino Acids
Mar 20, 2025
-
The Ambiguous Case Law Of Sines
Mar 20, 2025
-
The Ambiguous Case For The Law Of Sines
Mar 20, 2025
-
What Is The Definition Of Listening Style
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Are Covalent Bonds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
