Is A Polymer Of Amino Acids
Muz Play
Mar 20, 2025 · 6 min read
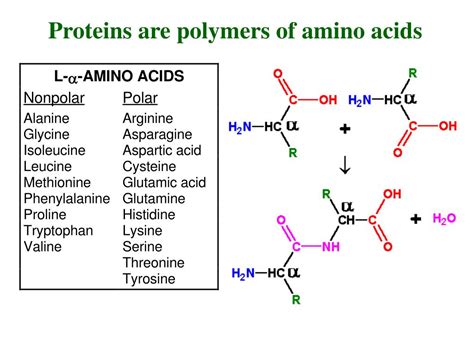
Table of Contents
Is a Polymer of Amino Acids: Delving Deep into the World of Proteins
Proteins are the workhorses of life, essential for virtually every biological process. From catalyzing reactions to providing structural support, their diverse functions stem from their unique molecular architecture. The simple answer to the question "Is a polymer of amino acids?" is a resounding yes. Proteins are indeed polymers, specifically polypeptides, constructed from a chain of amino acid monomers. This article delves deeper into this fundamental concept, exploring the intricacies of amino acids, the formation of peptide bonds, the levels of protein structure, and the remarkable diversity that arises from this seemingly simple building block.
Understanding Amino Acids: The Building Blocks of Proteins
Amino acids are organic molecules characterized by the presence of both an amino group (-NH₂) and a carboxyl group (-COOH) attached to a central carbon atom (the α-carbon). This α-carbon also carries a hydrogen atom and a unique side chain, often denoted as 'R'. This R-group is what differentiates the 20 standard amino acids found in proteins. These side chains exhibit a wide range of properties, including polarity, charge, size, and hydrophobicity, profoundly influencing the protein's overall structure and function.
The Diverse Properties of Amino Acid Side Chains
The hydrophobic amino acids, with their nonpolar side chains, tend to cluster in the protein's interior, away from the aqueous environment. Examples include alanine, valine, leucine, and isoleucine. Conversely, hydrophilic amino acids, with polar or charged side chains, are frequently found on the protein's surface, interacting with water molecules. Serine, threonine, tyrosine, and asparagine are examples of polar, uncharged amino acids. Charged amino acids, such as lysine, arginine, aspartate, and glutamate, contribute to the protein's overall charge and participate in electrostatic interactions.
Essential vs. Non-Essential Amino Acids
The human body can synthesize some amino acids, called non-essential amino acids, while others, termed essential amino acids, must be obtained from the diet. The essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. A deficiency in any essential amino acid can severely impact protein synthesis and overall health.
Peptide Bond Formation: Linking Amino Acids Together
The formation of a protein from its individual amino acid components involves a crucial process called dehydration synthesis. In this process, the carboxyl group of one amino acid reacts with the amino group of another amino acid, releasing a water molecule and forming a peptide bond. This peptide bond is an amide linkage, a strong covalent bond that links the amino acids together in a linear sequence. This sequence, determined by the genetic code, is known as the primary structure of the protein.
The Primary Structure: The Amino Acid Sequence
The primary structure dictates the higher-order structures of the protein. The specific order of amino acids is critical because even a single amino acid substitution can drastically alter the protein's properties and function. This is evident in genetic diseases like sickle cell anemia, where a single amino acid change in hemoglobin causes the protein to misfold and lead to serious health consequences.
Higher-Order Protein Structures: From Linear Chain to Functional Protein
The polypeptide chain formed by the peptide bonds doesn't exist as a simple, linear structure. Instead, it folds into complex three-dimensional arrangements, forming higher-order structures: secondary, tertiary, and quaternary.
Secondary Structure: Alpha-Helices and Beta-Sheets
The primary structure folds into local arrangements stabilized by hydrogen bonds between the backbone atoms. Two common secondary structures are alpha-helices and beta-sheets. Alpha-helices are coiled structures stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of an amino acid four residues down the chain. Beta-sheets are formed by hydrogen bonds between adjacent polypeptide chains or segments of a single chain arranged in a sheet-like structure.
Tertiary Structure: The Three-Dimensional Arrangement
The overall three-dimensional arrangement of the polypeptide chain, including its secondary structures, is known as the tertiary structure. This structure is stabilized by various interactions between the amino acid side chains, including:
- Hydrophobic interactions: Nonpolar side chains cluster together in the protein's core, away from water.
- Hydrogen bonds: Interactions between polar side chains and other polar groups.
- Ionic bonds (salt bridges): Interactions between oppositely charged side chains.
- Disulfide bonds: Covalent bonds between cysteine residues.
The tertiary structure is essential for the protein's function, creating specific binding sites and active sites for interactions with other molecules.
Quaternary Structure: Multiple Polypeptide Chains
Some proteins consist of multiple polypeptide chains, each with its own tertiary structure, associating to form a functional unit. This arrangement is known as the quaternary structure. Examples include hemoglobin, which is composed of four polypeptide subunits, and many enzymes that function as multimeric complexes. The interactions between the subunits are similar to those that stabilize the tertiary structure.
The Astonishing Diversity of Proteins: From Enzymes to Antibodies
The seemingly simple principle of linking amino acids together in various sequences leads to an incredible diversity of protein structures and functions. Proteins perform a vast array of roles, including:
- Enzymes: Catalyze biochemical reactions.
- Structural proteins: Provide support and shape to cells and tissues (e.g., collagen, keratin).
- Transport proteins: Carry molecules across cell membranes or throughout the body (e.g., hemoglobin).
- Motor proteins: Generate movement (e.g., myosin, kinesin).
- Hormones: Chemical messengers that regulate various physiological processes (e.g., insulin, growth hormone).
- Antibodies: Part of the immune system, recognizing and neutralizing foreign substances.
- Receptor proteins: Bind to specific molecules and trigger cellular responses.
The specific amino acid sequence, and hence the resulting three-dimensional structure, determines the function of each protein. The subtle differences in amino acid composition and arrangement allow for the vast functional diversity of proteins.
Protein Misfolding and Disease: The Importance of Proper Structure
The correct folding of proteins is crucial for their proper function. Errors in folding can lead to protein misfolding, causing a loss of function and potentially leading to various diseases. These diseases, known as proteinopathies, include:
- Alzheimer's disease: Associated with the accumulation of misfolded amyloid-beta proteins.
- Parkinson's disease: Linked to the aggregation of misfolded alpha-synuclein.
- Huntington's disease: Caused by the misfolding of huntingtin protein.
- Cystic fibrosis: Results from the misfolding of the cystic fibrosis transmembrane conductance regulator (CFTR) protein.
These diseases highlight the critical importance of proper protein folding and the devastating consequences of protein misfolding. Research into protein folding and misfolding is ongoing, with the aim of developing therapies to prevent or treat these diseases.
Conclusion: The Remarkable World of Protein Polymers
In conclusion, proteins are indeed polymers of amino acids, and the diversity and complexity arising from this simple principle are truly remarkable. The linear sequence of amino acids, the intricate folding patterns, and the diverse interactions between amino acid side chains all contribute to the unique properties and functions of proteins. Understanding the structure and function of proteins is essential for comprehending the intricacies of life and developing treatments for various diseases. Further research continues to unravel the secrets of protein folding, dynamics, and interactions, revealing further complexities and providing new avenues for therapeutic interventions. The study of proteins remains a vibrant and crucial field in biology and medicine, promising exciting advancements in the years to come.
Latest Posts
Latest Posts
-
Chemistry The Molecular Nature Of Matter And Change 10th Edition
Mar 21, 2025
-
Which Element Has The Highest Ionization Energy
Mar 21, 2025
-
What Is The Difference Between Energy And Matter
Mar 21, 2025
-
Find The Tangential And Normal Components Of The Acceleration Vector
Mar 21, 2025
-
Which Objective Lens Requires Oil To Be Applied
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Is A Polymer Of Amino Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
