What Is The Difference Between Energy And Matter
Muz Play
Mar 21, 2025 · 6 min read
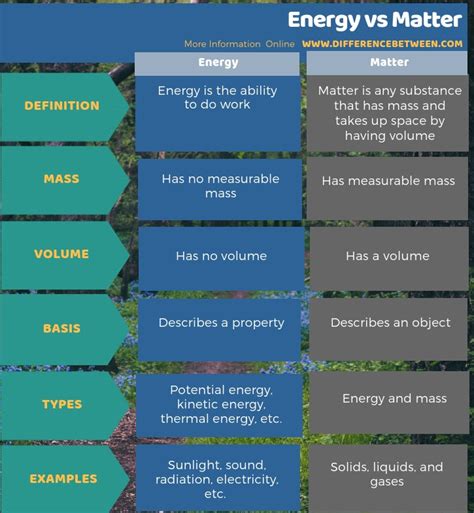
Table of Contents
What's the Difference Between Energy and Matter? A Deep Dive
The seemingly simple question, "What's the difference between energy and matter?" belies a profound and fascinating exploration into the fundamental building blocks of our universe. While seemingly distinct, the relationship between energy and matter is far more intricate than a simple dichotomy. This article will delve deep into the differences, exploring the historical context, scientific breakthroughs, and the implications of their interconnectedness.
Defining Energy and Matter: A Historical Perspective
The concepts of energy and matter have evolved significantly throughout scientific history. Initially, they were considered separate and distinct entities. Matter, in its classical definition, was anything that occupied space and possessed mass. Energy, on the other hand, was defined as the capacity to do work. This distinction worked well for explaining many everyday phenomena. However, groundbreaking discoveries in the 20th century fundamentally reshaped our understanding.
The Newtonian View: Distinct Entities
Newtonian physics, dominant for centuries, treated energy and matter as separate entities governed by different laws. Newton's laws of motion described the behavior of matter, while the principles of mechanics dealt with energy transfer and transformations. This separation, while useful for many applications, proved inadequate when confronting the complexities of the subatomic world.
The Einsteinian Revolution: E=mc² and the Interconnectedness
Albert Einstein's theory of special relativity revolutionized our comprehension of energy and matter with his iconic equation, E=mc². This equation revealed a profound connection: energy and mass are interchangeable. Mass (m) is a form of energy (E), and a small amount of mass can be converted into a tremendous amount of energy (c² represents the speed of light squared, a very large number).
This equation dramatically altered the classical view. It wasn't just that energy could be converted into matter, but that they were fundamentally different forms of the same thing. Nuclear reactions, like fission and fusion, provide compelling evidence for this interconnectedness.
Key Differences: Beyond E=mc²
While E=mc² highlights their fundamental relationship, several key distinctions remain between energy and matter:
1. Mass and Inertia: The Defining Property of Matter
A core difference lies in mass and inertia. Matter possesses mass, a measure of its resistance to acceleration (inertia). Energy, in its various forms, does not possess rest mass. While it can have momentum (energy in motion), it doesn't exhibit inertia in the same way as matter. Photons, for instance, are massless particles of light that carry energy.
2. Spatial Occupancy: Matter's Exclusive Domain
Matter occupies physical space. You can touch it, feel it, and see it. Energy, conversely, doesn't occupy space in the same way. While energy can be localized (like the energy stored in a battery), it doesn't have the physical extent of matter. Consider a beam of light: the energy is concentrated in the beam, but the beam itself isn't a physical object in the same sense that a solid object is.
3. Interaction with Gravity: A Differentiating Force
Matter interacts with gravity through its mass. This gravitational interaction is responsible for the planet's orbit around the sun, among many other celestial phenomena. Energy interacts with gravity in a more subtle way. Gravitational fields can bend light (energy), but the interaction isn't as direct or prominent as the gravitational attraction between two massive objects.
4. Forms and Manifestations: Diverse Expressions
Both energy and matter exist in diverse forms. Matter can exist as solids, liquids, gases, and plasmas. Energy manifests as kinetic energy (energy of motion), potential energy (stored energy), thermal energy (heat), chemical energy (stored in chemical bonds), nuclear energy (stored within atomic nuclei), electromagnetic energy (light, radio waves, etc.), and more.
Exploring the Different Forms of Energy
The diversity of energy forms deserves a closer examination:
1. Kinetic Energy: Energy in Motion
Any object in motion possesses kinetic energy. This energy is directly proportional to its mass and the square of its velocity. A speeding car, a rolling ball, and even the atoms vibrating within a substance all possess kinetic energy.
2. Potential Energy: Stored Energy
Potential energy is energy stored due to an object's position or configuration. A stretched spring, a book held above the ground, and water behind a dam all possess potential energy. This energy can be released and converted into kinetic energy.
3. Thermal Energy: Heat Energy
Thermal energy is the internal energy of an object due to the kinetic energy of its constituent particles. The higher the temperature, the greater the thermal energy. Heat transfer involves the movement of thermal energy from a hotter object to a colder one.
4. Chemical Energy: Energy in Bonds
Chemical energy is stored within the bonds of molecules. When these bonds are broken or formed, energy is released or absorbed. This energy is crucial for driving metabolic processes in living organisms and powering chemical reactions.
5. Nuclear Energy: Energy from the Nucleus
Nuclear energy is the energy stored within the nucleus of an atom. Nuclear reactions, such as fission (splitting atoms) and fusion (combining atoms), release enormous amounts of energy, as demonstrated by nuclear power plants and the sun's energy production.
6. Electromagnetic Energy: Light and More
Electromagnetic energy encompasses various forms of radiation, including visible light, radio waves, microwaves, X-rays, and gamma rays. These are all forms of energy that travel as waves and are characterized by their frequency and wavelength.
The Interplay Between Energy and Matter: Examples
The interconnectedness of energy and matter is evident in various phenomena:
-
Nuclear reactions: As previously discussed, nuclear fission and fusion demonstrably convert mass into energy. A small amount of mass loss results in a tremendous release of energy.
-
Particle-antiparticle annihilation: When a particle and its antiparticle (e.g., an electron and a positron) collide, they annihilate each other, converting their mass entirely into energy in the form of photons.
-
Pair production: The reverse process, pair production, occurs when high-energy photons convert into a particle-antiparticle pair.
-
Photosynthesis: Plants convert light energy (electromagnetic energy) into chemical energy (stored in glucose molecules), demonstrating the transformation of energy into matter (or more accurately, into a form with increased mass).
-
Combustion: Burning fuel converts chemical energy into thermal energy and light.
Conclusion: A Unified Vision
The distinction between energy and matter, once a clear-cut separation, has blurred significantly. Einstein's E=mc² fundamentally altered our understanding, revealing the profound interconnectedness of these fundamental entities. While key differences remain concerning mass, inertia, and spatial occupancy, the ability of energy to transform into matter and vice versa underscores their fundamental unity. The continued exploration of these concepts remains a vital area of physics, constantly pushing the boundaries of our knowledge about the universe and its underlying principles. Understanding the intricate interplay between energy and matter is essential for grasping many fundamental processes in physics, chemistry, and biology.
Latest Posts
Latest Posts
-
How Does A Buffer Resist Change In Ph
Mar 21, 2025
-
1 2 Vs 1 4 Addition
Mar 21, 2025
-
Definition Of Cultural Lag In Sociology
Mar 21, 2025
-
Kinetic Energy Of A Spring Formula
Mar 21, 2025
-
Programmed And Non Programmed Decision Making
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Energy And Matter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
