How Does A Buffer Resist Change In Ph
Muz Play
Mar 21, 2025 · 6 min read
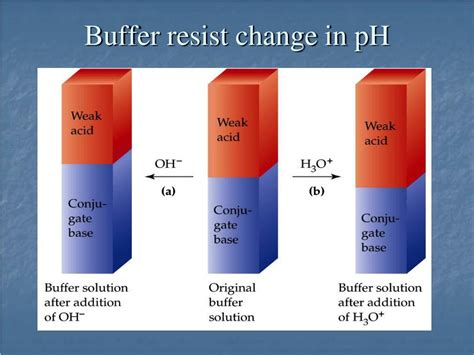
Table of Contents
- How Does A Buffer Resist Change In Ph
- Table of Contents
- How Does a Buffer Resist Change in pH?
- The Chemistry of Buffer Solutions: A Balancing Act
- The Henderson-Hasselbalch Equation: Quantifying Buffer Capacity
- Types of Buffer Systems: Diversity in Application
- 1. Acetate Buffer: A Classic Example
- 2. Phosphate Buffer: Biological Relevance
- 3. Carbonate Buffer: Maintaining Blood pH
- 4. Tris Buffer: A Versatile Choice
- Factors Affecting Buffer Effectiveness: Optimizing Performance
- 1. Concentration: More is Better (to a point)
- 2. Temperature: A Shifting Equilibrium
- 3. Ionic Strength: Influence on Activity
- Applications of Buffers: Across Diverse Fields
- 1. Biological Systems: Maintaining Homeostasis
- 2. Analytical Chemistry: Providing Stable Conditions
- 3. Pharmaceutical Industry: Formulating Medications
- 4. Food Industry: Controlling Food Quality
- 5. Environmental Monitoring: Measuring Water Quality
- Conclusion: The Indispensable Role of Buffers
- Latest Posts
- Latest Posts
- Related Post
How Does a Buffer Resist Change in pH?
Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. This crucial property makes them essential in numerous biological and chemical systems, maintaining a stable environment vital for various processes. Understanding how buffers achieve this resistance is fundamental to appreciating their significance. This article delves into the mechanisms behind buffer action, exploring the different types of buffers and their applications.
The Chemistry of Buffer Solutions: A Balancing Act
The ability of a buffer to resist pH change hinges on its composition: a weak acid and its conjugate base (or a weak base and its conjugate acid). This combination establishes an equilibrium that can absorb added H⁺ or OH⁻ ions, minimizing significant shifts in pH.
The Henderson-Hasselbalch Equation: Quantifying Buffer Capacity
The Henderson-Hasselbalch equation provides a quantitative description of the relationship between pH, pKa (the negative logarithm of the acid dissociation constant), and the concentrations of the weak acid (HA) and its conjugate base (A⁻):
pH = pKa + log([A⁻]/[HA])
This equation highlights the key factors determining a buffer's effectiveness:
- pKa: The closer the pKa of the weak acid is to the desired pH, the more effective the buffer will be. A pKa within ±1 pH unit of the target pH is generally considered optimal.
- [A⁻]/[HA] ratio: A ratio near 1 (equal concentrations of weak acid and conjugate base) provides the greatest buffering capacity. Significant deviations from this ratio reduce the buffer's effectiveness.
When a small amount of strong acid (e.g., HCl) is added to a buffer, the added H⁺ ions react with the conjugate base (A⁻), forming more weak acid (HA). This reaction consumes the added H⁺, preventing a drastic decrease in pH. Conversely, when a small amount of strong base (e.g., NaOH) is added, the added OH⁻ ions react with the weak acid (HA), forming more conjugate base (A⁻) and water. This reaction neutralizes the added OH⁻, preventing a significant pH increase.
The buffer's capacity to resist pH changes is limited. Adding excessive amounts of strong acid or base will eventually overwhelm the buffer, causing a dramatic pH shift. This limit is known as the buffer capacity.
Types of Buffer Systems: Diversity in Application
Numerous buffer systems exist, each tailored to specific pH ranges and applications. Some common examples include:
1. Acetate Buffer: A Classic Example
The acetate buffer system, consisting of acetic acid (CH₃COOH) and its conjugate base, acetate (CH₃COO⁻), is a widely used example. Acetic acid has a pKa of approximately 4.76, making this buffer effective in the pH range of 3.76 to 5.76. Its ease of preparation and relatively low cost contribute to its popularity in various laboratory settings.
2. Phosphate Buffer: Biological Relevance
Phosphate buffers, utilizing various phosphate species (e.g., H₂PO₄⁻ and HPO₄²⁻), are crucial in biological systems. Their pKa values span a range that encompasses physiological pH, making them ideal for maintaining the pH of cellular environments. The presence of phosphate in biological molecules further enhances its significance.
3. Carbonate Buffer: Maintaining Blood pH
The bicarbonate buffer system, comprising carbonic acid (H₂CO₃) and bicarbonate (HCO₃⁻), plays a vital role in maintaining the pH of blood. The lungs and kidneys work together to regulate the concentrations of these components, ensuring blood pH remains within a narrow, life-sustaining range (approximately 7.35-7.45). This system's effectiveness relies on the equilibrium between carbonic acid and dissolved carbon dioxide (CO₂), which can be expelled from the lungs.
4. Tris Buffer: A Versatile Choice
Tris buffer (tris(hydroxymethyl)aminomethane) is a widely used buffer in biochemistry and molecular biology. Its relatively high buffering capacity and lack of absorbance in the visible and UV regions make it suitable for various applications, including protein purification and enzymatic assays. However, the pKa of Tris is significantly affected by temperature, a factor that must be considered when using this buffer.
Factors Affecting Buffer Effectiveness: Optimizing Performance
Several factors can influence a buffer's ability to resist pH changes:
1. Concentration: More is Better (to a point)
Higher concentrations of the weak acid and conjugate base generally lead to greater buffer capacity. A more concentrated buffer can absorb larger amounts of added acid or base before a significant pH change occurs. However, excessively high concentrations may introduce other undesirable effects.
2. Temperature: A Shifting Equilibrium
Temperature affects the pKa of weak acids, potentially altering the buffer's effectiveness. As temperature changes, the equilibrium between the weak acid and its conjugate base shifts, impacting the pH. This effect is more pronounced for some buffers than others.
3. Ionic Strength: Influence on Activity
Ionic strength, a measure of the total concentration of ions in a solution, can influence the activity of the buffer components. High ionic strength can affect the equilibrium between the weak acid and its conjugate base, potentially reducing buffer effectiveness.
Applications of Buffers: Across Diverse Fields
The applications of buffer solutions span numerous fields:
1. Biological Systems: Maintaining Homeostasis
Buffers are crucial for maintaining the pH of biological systems, from the intracellular environment to the blood. They ensure optimal conditions for enzyme activity, protein folding, and other vital processes.
2. Analytical Chemistry: Providing Stable Conditions
In analytical chemistry, buffers provide stable pH conditions for various assays and titrations. They are essential for maintaining the accuracy and reproducibility of experimental results.
3. Pharmaceutical Industry: Formulating Medications
Buffers are frequently used in the formulation of pharmaceutical products to ensure drug stability and bioavailability. They help maintain the correct pH for drug absorption and prevent degradation.
4. Food Industry: Controlling Food Quality
Buffers are sometimes used in the food industry to maintain the pH of food products, influencing flavor, texture, and shelf life. They can help prevent microbial growth and maintain desired sensory attributes.
5. Environmental Monitoring: Measuring Water Quality
Buffers are used in environmental monitoring to maintain the pH of solutions used to measure water quality parameters. This ensures accurate and reliable results.
Conclusion: The Indispensable Role of Buffers
Buffer solutions play a vital role in a wide range of applications, all stemming from their remarkable ability to resist changes in pH. Understanding the principles behind buffer action—the Henderson-Hasselbalch equation, the interplay between weak acids and their conjugate bases, and the factors affecting buffer capacity—is essential for appreciating their significance. From maintaining the delicate pH balance of biological systems to enabling accurate measurements in analytical chemistry, buffers are an indispensable tool across various scientific and industrial fields. The continued development and application of new buffer systems will undoubtedly contribute to further advancements in numerous areas.
Latest Posts
Latest Posts
-
Definition Of Ion Product Constant For Water
Mar 23, 2025
-
Common Ion Effect Le Chatelier Principle
Mar 23, 2025
-
What Are The Functions Of State
Mar 23, 2025
-
Energized Electrons Leave Photosystem I And Are Used To Reduce
Mar 23, 2025
-
What Is The Color Of A Nucleus
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Does A Buffer Resist Change In Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
