Definition Of Ion Product Constant For Water
Muz Play
Mar 23, 2025 · 6 min read
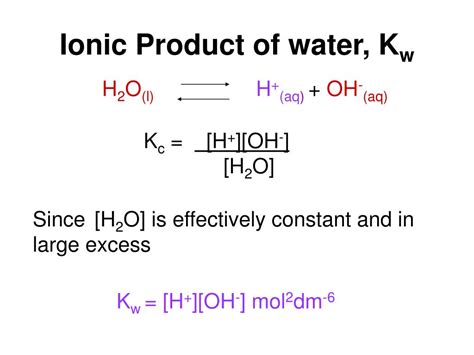
Table of Contents
The Ion Product Constant of Water: A Deep Dive
The ion product constant of water, often represented as K<sub>w</sub>, is a fundamental concept in chemistry, crucial for understanding aqueous solutions and their behavior. It quantifies the extent to which water self-ionizes, a process that has significant implications for acid-base chemistry and numerous other areas. This article will provide a comprehensive exploration of K<sub>w</sub>, including its definition, significance, variations with temperature and pressure, and applications in various chemical calculations.
Understanding Water's Self-Ionization
Pure water, despite its neutral pH, isn't simply H₂O. A small fraction of water molecules undergo a process called self-ionization or autoionization, where one water molecule donates a proton (H⁺) to another. This leads to the formation of hydronium ions (H₃O⁺) and hydroxide ions (OH⁻).
The reaction can be represented as:
2H₂O(l) ⇌ H₃O⁺(aq) + OH⁻(aq)
This equilibrium is dynamic, with water molecules constantly ionizing and re-forming. The double arrow indicates that the reaction proceeds in both directions simultaneously. At any given moment, a minuscule percentage of water molecules exist as ions.
Defining the Ion Product Constant (Kw)
The ion product constant for water (Kw) is the equilibrium constant for the self-ionization reaction. It's defined as the product of the concentrations of hydronium ions and hydroxide ions:
Kw = [H₃O⁺][OH⁻]
At 25°C (298 K), Kw has a value of approximately 1.0 × 10⁻¹⁴. This seemingly small value is significant because it reveals the low concentration of ions in pure water. The concentrations of both hydronium and hydroxide ions are equal in pure water, each being approximately 1.0 × 10⁻⁷ M. This equality is crucial for maintaining the neutral pH of 7.
Kw as an Equilibrium Constant
It's important to emphasize that K<sub>w</sub> is an equilibrium constant. Like all equilibrium constants, it's temperature-dependent. The value of 1.0 × 10⁻¹⁴ is only accurate at 25°C. Changes in temperature significantly alter the equilibrium position of the self-ionization reaction, thus affecting the value of K<sub>w</sub>.
Implications of Kw for pH and pOH
The K<sub>w</sub> is intrinsically linked to the concepts of pH and pOH. pH is defined as the negative logarithm (base 10) of the hydronium ion concentration:
pH = -log[H₃O⁺]
Similarly, pOH is the negative logarithm of the hydroxide ion concentration:
pOH = -log[OH⁻]
From the definition of K<sub>w</sub>, we can derive a crucial relationship between pH and pOH:
pH + pOH = 14 (at 25°C)
This relationship holds true only at 25°C, since K<sub>w</sub> changes with temperature. It allows us to calculate the pOH if we know the pH, and vice-versa.
Temperature Dependence of Kw
As mentioned earlier, K<sub>w</sub> is highly sensitive to temperature. The self-ionization of water is an endothermic process, meaning it absorbs heat. According to Le Chatelier's principle, increasing the temperature shifts the equilibrium to the right, favoring the formation of more hydronium and hydroxide ions. Consequently, K<sub>w</sub> increases with temperature.
Here's a summary of how K<sub>w</sub> changes with temperature:
- Below 25°C: K<sub>w</sub> is less than 1.0 × 10⁻¹⁴.
- At 25°C: K<sub>w</sub> is approximately 1.0 × 10⁻¹⁴.
- Above 25°C: K<sub>w</sub> is greater than 1.0 × 10⁻¹⁴.
The precise values of K<sub>w</sub> at different temperatures can be found in chemical handbooks and databases. The temperature dependence is crucial when performing calculations at temperatures other than 25°C. Failing to account for this temperature dependence can lead to significant errors in calculations.
Pressure Dependence of Kw
While the temperature dependence of K<sub>w</sub> is significant, the pressure dependence is relatively minor, especially within the range of pressures typically encountered in laboratory settings. However, at extremely high pressures, a slight increase in K<sub>w</sub> is observed.
Applications of Kw
The ion product constant of water finds extensive applications in various chemical calculations:
1. Calculating pH and pOH:
Knowing the concentration of either hydronium or hydroxide ions, along with the value of K<sub>w</sub>, allows us to calculate the other ion's concentration and subsequently determine the pH and pOH.
2. Determining the Acidity or Basicity of Solutions:
By comparing the concentrations of hydronium and hydroxide ions, we can determine whether a solution is acidic ( [H₃O⁺] > [OH⁻]), basic ([H₃O⁺] < [OH⁻]), or neutral ([H₃O⁺] = [OH⁻]).
3. Solving Equilibrium Problems:
K<sub>w</sub> is an essential component in solving equilibrium problems involving weak acids and weak bases. It's used in conjunction with the acid dissociation constant (Ka) or base dissociation constant (Kb) to determine the concentrations of various species in solution.
4. Understanding Buffer Solutions:
Buffer solutions resist changes in pH when small amounts of acid or base are added. The K<sub>w</sub> helps in understanding how buffer solutions work and in calculating their buffer capacity.
5. Analyzing Titration Curves:
In acid-base titrations, K<sub>w</sub> plays a crucial role in determining the equivalence point and the shape of the titration curve.
Kw and its Relationship to other Equilibrium Constants
The ion product constant of water is directly related to other important equilibrium constants in acid-base chemistry:
-
Acid dissociation constant (Ka): This constant represents the strength of an acid. A large Ka indicates a strong acid, while a small Ka indicates a weak acid. The relationship between Ka and Kw is often used to calculate the Kb (base dissociation constant) of the conjugate base.
-
Base dissociation constant (Kb): This constant represents the strength of a base. Similar to Ka, a large Kb indicates a strong base, while a small Kb indicates a weak base. Kw allows for the calculation of Ka for the conjugate acid.
Understanding these relationships is fundamental to comprehending the interplay between acids, bases, and their conjugate pairs in aqueous solutions.
Beyond the Basics: Isotopic Effects on Kw
While standard chemistry usually considers the self-ionization of H₂O, it's important to note that isotopic variations of water, like D₂O (heavy water), also undergo self-ionization, but with different K<sub>w</sub> values. The equilibrium constant for the self-ionization of heavy water is significantly lower than that for ordinary water, reflecting the differences in bond strengths and vibrational energies.
Conclusion
The ion product constant of water, K<sub>w</sub>, is a cornerstone of aqueous chemistry. Understanding its definition, temperature dependence, and applications is essential for mastering acid-base chemistry and solving numerous equilibrium problems. Its seemingly simple definition belies its immense importance in various scientific fields, ranging from environmental chemistry to biochemistry and beyond. As we've seen, its precise value is highly sensitive to temperature, and careful attention to this detail is critical for accurate calculations and a deeper understanding of the chemical processes occurring in aqueous solutions. The exploration of K<sub>w</sub> serves as a powerful illustration of the interconnectedness of fundamental concepts in chemistry and their significance in numerous real-world applications.
Latest Posts
Latest Posts
-
Which Of The Following Is A Simple Definition Of Reduction
Mar 24, 2025
-
What Are The Characteristics Of The State
Mar 24, 2025
-
Buffer Acetic Acid And Sodium Acetate
Mar 24, 2025
-
A Complicated Molecule Derived Or Made From Lipids
Mar 24, 2025
-
Periodic Table Labeled With Valence Electrons
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Definition Of Ion Product Constant For Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
