Buffer Acetic Acid And Sodium Acetate
Muz Play
Mar 24, 2025 · 6 min read
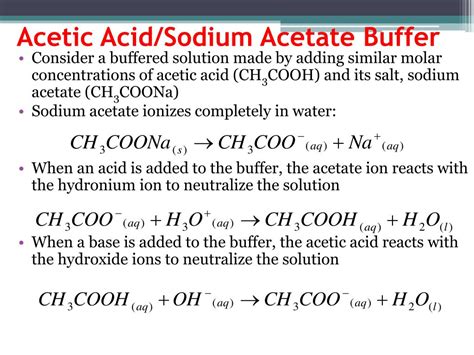
Table of Contents
Understanding the Power of Buffer Solutions: A Deep Dive into Acetic Acid and Sodium Acetate
Buffer solutions are crucial in various chemical and biological systems, maintaining a relatively stable pH even when small amounts of acid or base are added. One of the most common and well-understood buffer systems is the acetic acid/sodium acetate buffer. This article delves deep into the chemistry behind this buffer, exploring its properties, applications, and limitations.
What is a Buffer Solution?
A buffer solution, or simply a buffer, is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid. Its primary function is to resist changes in pH upon the addition of small amounts of a strong acid or strong base. This resistance to pH change is vital in many applications where a stable pH environment is crucial.
The effectiveness of a buffer is determined by its buffer capacity, which represents the amount of acid or base that can be added before a significant change in pH occurs. The buffer capacity is highest when the concentrations of the weak acid and its conjugate base are equal.
The Acetic Acid/Sodium Acetate Buffer System
The acetic acid/sodium acetate buffer is a classic example of a weak acid-conjugate base buffer system. Acetic acid (CH₃COOH), a weak acid, partially dissociates in water, releasing a small amount of hydronium ions (H₃O⁺) and acetate ions (CH₃COO⁻). Sodium acetate (CH₃COONa), a salt, completely dissociates in water, providing a source of acetate ions.
The equilibrium reaction governing this buffer system is:
CH₃COOH(aq) + H₂O(l) ⇌ CH₃COO⁻(aq) + H₃O⁺(aq)
The presence of both acetic acid and acetate ions allows the buffer to respond to the addition of either acid or base.
How the Buffer Works: Resisting pH Changes
-
Addition of a Strong Acid (e.g., HCl): When a strong acid is added, the hydronium ions react with the acetate ions to form acetic acid:
CH₃COO⁻(aq) + H₃O⁺(aq) → CH₃COOH(aq) + H₂O(l)
This reaction consumes the added hydronium ions, minimizing the increase in pH.
-
Addition of a Strong Base (e.g., NaOH): When a strong base is added, the hydroxide ions react with the acetic acid to form acetate ions and water:
CH₃COOH(aq) + OH⁻(aq) → CH₃COO⁻(aq) + H₂O(l)
This reaction consumes the added hydroxide ions, minimizing the decrease in pH.
The Henderson-Hasselbalch equation is a useful tool for calculating the pH of a buffer solution:
pH = pKa + log([A⁻]/[HA])
where:
- pH is the pH of the buffer solution
- pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid (acetic acid in this case)
- [A⁻] is the concentration of the conjugate base (acetate ion)
- [HA] is the concentration of the weak acid (acetic acid)
The pKa of acetic acid is approximately 4.76. When the concentrations of acetic acid and acetate are equal ([A⁻]/[HA] = 1), the pH of the buffer is equal to the pKa.
Preparation of Acetic Acid/Sodium Acetate Buffer
Preparing an acetic acid/sodium acetate buffer involves carefully mixing specific amounts of acetic acid and sodium acetate to achieve the desired pH. The exact amounts will depend on the desired pH and buffer capacity. One common approach is to use a stock solution of acetic acid and a stock solution of sodium acetate, then mixing them in appropriate ratios.
Accurate measurements and careful mixing are crucial to ensure the buffer's effectiveness. The use of calibrated instruments, such as pH meters, is essential for verifying the final pH of the prepared buffer.
Factors Affecting Buffer Capacity
Several factors influence the effectiveness of the acetic acid/sodium acetate buffer:
-
Concentrations of Acetic Acid and Sodium Acetate: Higher concentrations lead to a higher buffer capacity. A higher concentration means a larger amount of acid or base can be absorbed before a significant change in pH occurs.
-
Ratio of Acetic Acid to Sodium Acetate: The buffer capacity is maximized when the concentrations of acetic acid and sodium acetate are equal. Deviation from this ideal ratio reduces the buffer capacity.
-
Temperature: Temperature affects the pKa of acetic acid, and therefore the pH of the buffer. Precise temperature control might be necessary for applications requiring high precision.
Applications of Acetic Acid/Sodium Acetate Buffer
The acetic acid/sodium acetate buffer finds widespread use in various applications due to its relatively simple preparation and its stable pH within a specific range. Some key applications include:
1. Biochemical Research and Experiments
The buffer's ability to maintain a stable pH is essential in many biological and biochemical experiments. Enzyme reactions, cell cultures, and protein purification often require a specific pH range, and the acetic acid/sodium acetate buffer can effectively provide this controlled environment. Its pH range around 4-5 is suitable for certain enzymatic reactions and maintaining the stability of biological molecules.
2. Food Preservation and Processing
In the food industry, the buffer might be used to control the pH of food products. This helps prevent microbial growth and maintains the desired sensory properties of the food. The range around 4-5 inhibits the growth of many spoilage microorganisms.
3. Pharmaceutical Applications
In pharmaceutical formulations, buffers are used to maintain the stability and efficacy of drugs. The acetic acid/sodium acetate buffer might be incorporated into drug formulations to ensure the drug's stability and prevent degradation. Precise pH control is critical to ensure the drug's bioavailability and effectiveness.
4. Analytical Chemistry
In analytical chemistry, the buffer might be used as a solvent or a component of a reagent. Maintaining a constant pH during certain titrations or analytical procedures is crucial for accurate results. The buffering capacity ensures that any changes caused during the reaction remain minimal.
5. Textile Industry
The buffer plays a role in dyeing and finishing processes in the textile industry. Maintaining a consistent pH ensures the dye fixation and proper coloration of fabrics.
Limitations of the Acetic Acid/Sodium Acetate Buffer
While the acetic acid/sodium acetate buffer is versatile, it has limitations:
-
Limited pH Range: This buffer is most effective within a pH range of approximately 3.76 to 5.76 (pKa ± 1). Outside this range, its buffering capacity decreases significantly.
-
Temperature Sensitivity: The pKa of acetic acid is temperature-dependent. Significant temperature fluctuations can affect the buffer's pH.
-
Ionic Strength: The presence of other ions in the solution can alter the ionic strength, which can indirectly affect the buffer's pH.
-
Not Suitable for all Applications: The acetic acid/sodium acetate buffer is not appropriate for all applications requiring a buffer. Its pH range might not be suitable for reactions or processes requiring a pH outside its effective range.
Conclusion: A Versatile and Reliable Buffer
The acetic acid/sodium acetate buffer is a valuable tool in various fields due to its readily available components, ease of preparation, and effective buffering capacity within its operational pH range. Understanding its properties, limitations, and applications allows researchers and practitioners to leverage its strengths effectively, contributing to advancements in various scientific and industrial domains. By carefully considering the factors influencing its buffer capacity and selecting the appropriate concentration ratio, researchers can create a stable environment crucial for successful experiments and processes. While not a universal solution for all pH control needs, its importance in maintaining a stable environment in specific applications remains undeniable.
Latest Posts
Latest Posts
-
What Happens To Electrons In Metallic Bonding
Mar 26, 2025
-
Label The Types Of Intercellular Junctions
Mar 26, 2025
-
Is Soil Renewable Or Nonrenewable Resource
Mar 26, 2025
-
Are The Initial Velocities On An Uncompetitive Inhibitor The Same
Mar 26, 2025
-
What Is The Relationship Between Cells And Tissues
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Buffer Acetic Acid And Sodium Acetate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
