Periodic Table Labeled With Valence Electrons
Muz Play
Mar 24, 2025 · 7 min read
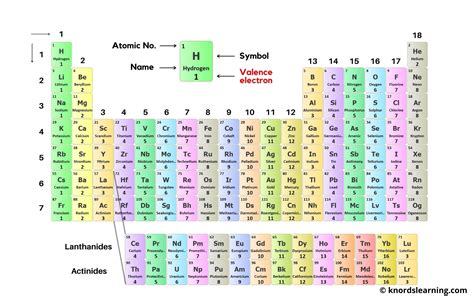
Table of Contents
The Periodic Table: A Deep Dive into Valence Electrons
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding this organization is crucial to comprehending chemical reactions and the behavior of matter. Central to this understanding is the concept of valence electrons, the outermost electrons in an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of compounds it can form. This article provides a comprehensive exploration of the periodic table, focusing specifically on the role and distribution of valence electrons.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (also called the valence shell) of an atom. These electrons are the furthest from the atom's nucleus and experience the weakest attraction to it. This makes them readily available to participate in chemical bonds with other atoms. The number of valence electrons an atom possesses largely dictates its chemical behavior. Atoms tend to react in ways that achieve a stable electron configuration, often resembling the noble gases with their full valence shells (eight electrons, except for helium with two).
How to Determine Valence Electrons
Determining the number of valence electrons for an element can be done in several ways:
-
Using the Group Number (for main group elements): For main group elements (Groups 1, 2, and 13-18), the group number generally corresponds to the number of valence electrons. For example, elements in Group 1 (alkali metals) have one valence electron, elements in Group 2 (alkaline earth metals) have two, and so on. Note that this rule doesn't apply to transition metals (Groups 3-12).
-
Using the Electron Configuration: The electron configuration shows the arrangement of electrons in an atom's shells and subshells. The valence electrons are those in the highest energy level (principal quantum number). For instance, the electron configuration of oxygen is 1s²2s²2p⁴. The highest energy level is n=2, containing 2s²2p⁴ electrons, totaling six valence electrons.
-
Using the Periodic Table Visualization: While not a direct calculation, a well-labeled periodic table can visually represent valence electron trends. Understanding the periodic table's structure, and knowing the general trends in valence electron numbers across periods and down groups, allows for quick estimations.
The Periodic Table and Valence Electron Trends
The periodic table itself is a visual representation of valence electron trends. The arrangement of elements reflects the recurring patterns in their electronic structures and, consequently, their chemical properties.
Periods and Valence Electrons
As you move across a period (row) from left to right, the number of valence electrons generally increases. This is because each element in a period adds an electron to the same outermost shell. For example, in the second period (Li to Ne), lithium has one valence electron, beryllium has two, boron has three, and so on, until neon has eight (a full octet).
Groups and Valence Electrons
Elements within the same group (column) share the same number of valence electrons. This is why elements in a group exhibit similar chemical properties. For example, all alkali metals (Group 1) readily lose one electron to form a +1 ion, while all halogens (Group 17) readily gain one electron to form a -1 ion.
Transition Metals and Valence Electrons
Transition metals (Groups 3-12) are an exception to the simple group number-valence electron relationship. Their valence electrons can occupy both the outermost s and d subshells. This results in more complex and varied chemical behavior, with multiple oxidation states being common. Predicting the exact number of valence electrons for a transition metal can be more challenging and often depends on the specific compound or reaction.
Valence Electrons and Chemical Bonding
Valence electrons are the driving force behind chemical bonding. Atoms interact to achieve a more stable electron configuration, typically by attaining a full valence shell (octet rule). There are three main types of chemical bonds:
Ionic Bonds
Ionic bonds form when one atom transfers one or more valence electrons to another atom. This creates ions – charged particles – with one atom becoming positively charged (cation) and the other becoming negatively charged (anion). The electrostatic attraction between these oppositely charged ions forms the ionic bond. This is common between elements with significantly different electronegativities, such as metals (low electronegativity, tend to lose electrons) and nonmetals (high electronegativity, tend to gain electrons). For example, the formation of sodium chloride (NaCl) involves sodium (Na) losing one valence electron to chlorine (Cl), resulting in Na⁺ and Cl⁻ ions.
Covalent Bonds
Covalent bonds form when atoms share valence electrons. This sharing allows both atoms to achieve a more stable electron configuration. Covalent bonds are common between nonmetals, where the electronegativity difference is relatively small. For example, the oxygen molecule (O₂) involves two oxygen atoms sharing two pairs of valence electrons. The number of shared electron pairs determines the bond order (single, double, or triple bonds).
Metallic Bonds
Metallic bonds are found in metals and involve the delocalization of valence electrons. These electrons are not associated with any particular atom but rather move freely throughout the metal lattice. This "sea" of delocalized electrons accounts for the characteristic properties of metals like electrical and thermal conductivity, malleability, and ductility.
Visualizing Valence Electrons on the Periodic Table
A visually enhanced periodic table can be a powerful tool for understanding valence electron trends. A labeled periodic table should clearly indicate:
- Group Numbers: These are essential for determining the valence electrons of main group elements.
- Element Symbols and Names: Clearly identifying each element is crucial.
- Electron Configuration (abbreviated): Showing the electron configuration, at least for the valence shell, provides a deeper understanding of electron arrangement.
- Valence Electron Count: Explicitly stating the number of valence electrons for each element significantly aids comprehension.
- Color-coding by Valence Electrons: Using different colors to represent elements with different numbers of valence electrons can create a visually striking representation highlighting the periodic trends.
- Highlighting Noble Gases: Emphasizing the noble gases with their full valence shells helps illustrate the stability these configurations represent.
Practical Applications of Understanding Valence Electrons
Understanding valence electrons is not just a theoretical exercise; it has numerous practical applications in various fields:
- Predicting Chemical Reactions: Knowing the number of valence electrons allows chemists to predict how atoms will interact and what types of bonds will form. This is crucial in designing and synthesizing new materials and understanding chemical reactions in various industrial processes.
- Material Science: The properties of materials are largely determined by the bonding between their constituent atoms. Understanding valence electrons helps in designing materials with specific properties, such as strength, conductivity, or reactivity.
- Biochemistry: Valence electrons play a critical role in biological molecules, such as proteins and DNA. Understanding how these electrons participate in bonding and interactions is crucial for understanding biological processes.
- Electronics: The behavior of semiconductors and conductors is directly related to the number and arrangement of valence electrons. This understanding is fundamental to the development of electronic devices.
Conclusion
The periodic table, with its systematic organization of elements, is an invaluable tool for understanding the behavior of matter. Focusing on the crucial role of valence electrons enhances this understanding significantly. By knowing how to determine and visualize valence electron trends, we gain valuable insight into chemical bonding, reactivity, and the properties of elements and their compounds. This knowledge forms the basis for many advancements in chemistry, materials science, and various other scientific and technological fields. A well-labeled periodic table, clearly showing valence electron counts and trends, serves as a powerful visual aid in mastering this fundamental concept of chemistry. Continuing to explore and understand the intricate relationship between the periodic table and valence electrons will undoubtedly lead to further breakthroughs in our comprehension of the natural world.
Latest Posts
Latest Posts
-
When A Substance In A Reaction Is Oxidized It
Mar 26, 2025
-
What Happens To Electrons In Metallic Bonding
Mar 26, 2025
-
Label The Types Of Intercellular Junctions
Mar 26, 2025
-
Is Soil Renewable Or Nonrenewable Resource
Mar 26, 2025
-
Are The Initial Velocities On An Uncompetitive Inhibitor The Same
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Labeled With Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
