Common Ion Effect Le Chatelier Principle
Muz Play
Mar 23, 2025 · 6 min read
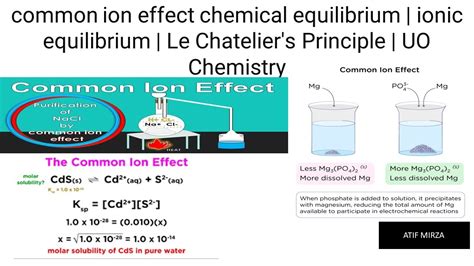
Table of Contents
Common Ion Effect and Le Chatelier's Principle: A Comprehensive Guide
The common ion effect is a phenomenon observed in chemistry that describes the decrease in the solubility of a sparingly soluble salt when a soluble salt containing a common ion is added to the solution. This effect is a direct consequence of Le Chatelier's principle, a fundamental concept in chemical equilibrium. Understanding both is crucial for anyone working with chemical solutions, from students learning equilibrium chemistry to professionals in analytical chemistry and various other fields. This comprehensive guide will explore these concepts in detail, providing examples and applications.
Le Chatelier's Principle: The Foundation
Before delving into the common ion effect, it's essential to understand Le Chatelier's principle. This principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. These changes can include:
- Changes in concentration: Adding or removing reactants or products.
- Changes in temperature: Increasing or decreasing the temperature of the system.
- Changes in pressure: Increasing or decreasing the pressure (primarily affecting gaseous systems).
The system will respond to minimize the effect of the imposed change and re-establish equilibrium, although the equilibrium constant itself may change with temperature. Let's consider a simple reversible reaction:
A + B ⇌ C + D
If we add more of reactant A, the system will shift to the right, producing more C and D to consume the excess A and restore equilibrium. Conversely, removing product C will cause the equilibrium to shift to the right, attempting to replenish the lost C. This fundamental principle is the key to understanding the common ion effect.
Temperature's Impact on Equilibrium
Temperature's role in equilibrium is unique because it directly affects the equilibrium constant (K). For exothermic reactions (those that release heat), increasing the temperature shifts the equilibrium to the left (favoring reactants), while decreasing the temperature shifts it to the right (favoring products). The opposite is true for endothermic reactions (those that absorb heat). This is because altering the temperature changes the relative energies of reactants and products, impacting the equilibrium position.
The Common Ion Effect: A Deeper Dive
Now, let's focus on the common ion effect. This effect specifically deals with the solubility of sparingly soluble salts. A sparingly soluble salt is one that dissolves only slightly in water, resulting in a relatively low concentration of its ions in solution. The common ion effect states that the solubility of a sparingly soluble salt decreases when a soluble salt containing a common ion is added to the solution.
Consider the solubility equilibrium of silver chloride (AgCl):
AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
Silver chloride is sparingly soluble. Its solubility product constant, Ksp, is a measure of its solubility, defined as:
Ksp = [Ag⁺][Cl⁻]
If we add a soluble chloride salt, such as sodium chloride (NaCl), to a saturated solution of AgCl, the concentration of the chloride ion ([Cl⁻]) increases. According to Le Chatelier's principle, the system will shift to the left to relieve the stress caused by the increased [Cl⁻]. This shift means that more AgCl will precipitate out of solution, thus decreasing the solubility of AgCl. The overall concentration of Ag⁺ ions will decrease, demonstrating the common ion effect.
Quantifying the Common Ion Effect
The impact of the common ion effect can be quantified using the solubility product constant (Ksp). Let's say we have a saturated solution of AgCl, and then we add a soluble salt containing a common ion. The common ion effect is noticeable in calculations of solubility in the presence of a common ion. In a solution with only AgCl, the concentrations of Ag⁺ and Cl⁻ are equal, and the solubility of AgCl is equal to the square root of Ksp. However, when the common ion (e.g., Cl⁻ from NaCl) is added, the solubility significantly decreases, becoming less than the square root of Ksp.
Examples of Common Ion Effect
The common ion effect finds numerous applications in various fields:
-
Qualitative analysis: In qualitative inorganic analysis, the common ion effect is often used to selectively precipitate ions from a mixture. By adding a common ion, the solubility of a specific ion can be drastically reduced, allowing for its separation from other ions.
-
Solubility control: The common ion effect is crucial in controlling the solubility of compounds in different applications, such as in pharmaceutical formulations or industrial processes. Adjusting the concentration of the common ion allows for precise manipulation of the solubility of the desired compound.
Applications and Significance
The common ion effect and Le Chatelier's principle are not just theoretical concepts; they have practical applications across multiple domains:
Analytical Chemistry
The common ion effect is frequently used in gravimetric analysis, a quantitative method for determining the amount of a substance by weighing a precipitate. By adding a common ion, the precipitation of the analyte is enhanced, leading to more complete and accurate results.
Environmental Chemistry
Understanding the common ion effect is essential in environmental remediation. For example, it can be applied to predict and control the solubility of heavy metal ions in contaminated soil or water. By adjusting the concentration of common ions, the mobility and bioavailability of these toxic metals can be managed.
Pharmaceutical Sciences
In pharmaceutical science, the common ion effect influences the solubility and bioavailability of drugs. Adjusting the ionic strength of a solution through the addition of common ions can be used to modify drug release profiles and improve patient outcomes.
Industrial Processes
Various industrial processes rely on controlling solubility. The common ion effect can be used to optimize the precipitation of desired products or to prevent unwanted precipitation, thus impacting efficiency and product purity.
Further Considerations
While the common ion effect primarily focuses on the impact of a common ion on solubility, it is important to note that other factors such as temperature, pH, and complex ion formation can also influence solubility. These factors should be considered for a complete understanding of solubility behavior. The presence of other ions that do not share a common ion may also have minor effects through ionic strength changes, which affects activity coefficients. However, the effect of the common ion will be much more significant.
Conclusion
The common ion effect, a direct consequence of Le Chatelier's principle, is a fundamental concept in chemistry with broad-reaching applications. Understanding this effect is crucial for interpreting and controlling the solubility of sparingly soluble salts in various contexts. From analytical chemistry to environmental science and pharmaceutical development, the principle serves as a powerful tool for manipulating and predicting the behavior of chemical systems. By mastering the common ion effect and its underlying principles, one can effectively address various challenges in diverse scientific and industrial applications. The interplay between these two concepts highlights the interconnectedness of chemical equilibrium and its importance in shaping the behavior of chemical systems. This comprehensive understanding provides a solid foundation for advanced studies in chemistry and related fields.
Latest Posts
Latest Posts
-
How Many People Is Considered A Group
Mar 24, 2025
-
Group 11 Of The Periodic Table
Mar 24, 2025
-
R We Supposed To Memorize Electronegativity Values
Mar 24, 2025
-
An Intensive Property Of A Substance Is
Mar 24, 2025
-
Diffusion Of Water Across A Selectively Permeable Membrane
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Common Ion Effect Le Chatelier Principle . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
