R We Supposed To Memorize Electronegativity Values
Muz Play
Mar 24, 2025 · 5 min read
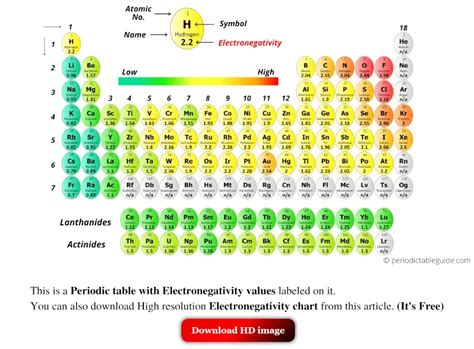
Table of Contents
Are We Supposed to Memorize Electronegativity Values? A Comprehensive Guide
The question of whether to memorize electronegativity values is a common one among chemistry students. The short answer is: it depends. While rote memorization isn't strictly necessary for all aspects of chemistry, understanding the concept of electronegativity and its trends is crucial. This comprehensive guide explores electronegativity, its applications, and effective strategies for mastering this important chemical concept without necessarily resorting to memorization.
Understanding Electronegativity: More Than Just Numbers
Electronegativity, represented by the Greek letter χ (chi), is a fundamental chemical property that describes an atom's ability to attract shared electrons in a chemical bond. It's not a directly measurable quantity like mass or charge, but rather a relative measure derived from experimental observations and theoretical calculations. Higher electronegativity values indicate a stronger ability to attract electrons.
Instead of focusing on memorizing specific numbers, concentrate on understanding the periodic trends of electronegativity. This provides a much more powerful and applicable understanding than simply memorizing a list of values.
Key Periodic Trends in Electronegativity:
-
Increases across a period (left to right): As you move across a period of the periodic table, the number of protons in the nucleus increases while the principal quantum number remains constant. This leads to a stronger pull on electrons, resulting in higher electronegativity. The effective nuclear charge increases, pulling electrons closer to the nucleus.
-
Decreases down a group (top to bottom): Going down a group, the atomic radius increases. The outer electrons are further from the nucleus, shielded by inner electrons. This reduces the effective nuclear charge experienced by the valence electrons, hence lower electronegativity. Increased shielding and distance weaken the attraction to the nucleus.
These trends allow you to predict relative electronegativities without resorting to memorization. For example, you can confidently state that fluorine (F) is more electronegative than oxygen (O) because it lies to the right and above it on the periodic table.
Applications of Electronegativity: Beyond Simple Memorization
Understanding electronegativity is critical for predicting and explaining various chemical phenomena. Simply memorizing values doesn't convey the underlying principles. Here are some key applications:
1. Predicting Bond Polarity:
The difference in electronegativity between two atoms involved in a bond determines its polarity.
-
Nonpolar covalent bonds: Occur when the electronegativity difference is small (generally less than 0.5). Electrons are shared relatively equally. Examples include bonds between two identical atoms (e.g., H-H, Cl-Cl).
-
Polar covalent bonds: Occur when the electronegativity difference is moderate (generally between 0.5 and 1.7). Electrons are shared unequally, creating a dipole moment with a slightly positive (δ+) and slightly negative (δ-) end. Examples include H-Cl, C-O.
-
Ionic bonds: Occur when the electronegativity difference is large (generally greater than 1.7). Electrons are essentially transferred from the less electronegative atom to the more electronegative atom, resulting in the formation of ions. Examples include NaCl, MgO.
Understanding these ranges allows you to predict the type of bond and thus, the properties of the molecule. This is far more valuable than memorizing electronegativity values themselves.
2. Predicting Molecular Geometry and Dipole Moments:
Electronegativity plays a crucial role in determining the distribution of electron density within a molecule. This, in turn, affects its molecular geometry and overall dipole moment. A molecule with polar bonds may have a net dipole moment if the individual bond dipoles don't cancel each other out due to the molecular geometry (e.g., water, H₂O).
3. Understanding Chemical Reactivity:
Electronegativity influences the reactivity of atoms and molecules. More electronegative atoms tend to attract electrons more strongly, making them better oxidizing agents (they readily accept electrons). Less electronegative atoms are better reducing agents (they readily donate electrons).
4. Predicting Acid-Base Strength:
Electronegativity helps predict the strength of acids and bases. In general, acids with more electronegative atoms attached to the acidic hydrogen are stronger because the electronegative atom helps stabilize the resulting conjugate base.
Effective Strategies for Mastering Electronegativity:
Rather than focusing on memorization, concentrate on these strategies:
-
Focus on Trends: Master the periodic trends of electronegativity. Understanding why electronegativity changes across periods and down groups is more valuable than remembering specific values.
-
Use the Periodic Table as a Guide: The periodic table itself serves as a valuable tool. By understanding the trends, you can estimate the relative electronegativities of elements.
-
Practice Predicting Bond Polarity: Work through numerous examples of predicting bond polarity based on electronegativity differences. This reinforces the concept and helps you develop intuition.
-
Visual Aids and Mnemonics: Use visual aids like diagrams showing electronegativity trends on the periodic table. Consider creating your own mnemonics to help remember the relative electronegativities of common elements. But remember, understanding the trends is key.
-
Solve Problems: Practice problems focusing on bond polarity, molecular geometry, and chemical reactivity. This is the most effective way to solidify your understanding.
When Memorization Might Be Helpful:
While not strictly necessary, memorizing the electronegativity values of a few highly common elements might be helpful for speed in some problem-solving situations. These would likely include:
- Fluorine (F): The most electronegative element.
- Oxygen (O): A highly electronegative element.
- Nitrogen (N): Moderately electronegative.
- Chlorine (Cl): Highly electronegative.
- Hydrogen (H): Moderately low electronegativity.
- Carbon (C): Moderately low electronegativity.
However, even this limited memorization is secondary to understanding the underlying trends and applying them consistently.
Conclusion: Understanding Trumps Memorization
In conclusion, while you might choose to memorize a few key electronegativity values for efficiency, the primary focus should be on understanding the concept of electronegativity, its periodic trends, and its numerous applications in chemistry. Mastering these aspects will empower you to solve problems, predict chemical behavior, and develop a deeper understanding of chemical bonding and reactivity than simple memorization ever could. Focus on understanding the why, not just the what. This approach is significantly more valuable and will serve you far better in your chemistry studies.
Latest Posts
Latest Posts
-
What Is The Ph Of Salt
Mar 28, 2025
-
Activity Series Of Metals And Non Metals
Mar 28, 2025
-
How Many Unpaired Electrons Does Carbon Have
Mar 28, 2025
-
What Is Damping In A Wave
Mar 28, 2025
-
How To Calculate Heat Of Dissolution Without Temperature
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about R We Supposed To Memorize Electronegativity Values . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
