What Is The Ph Of Salt
Muz Play
Mar 28, 2025 · 6 min read
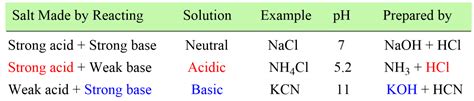
Table of Contents
What is the pH of Salt? Understanding Salinity and its Impact on pH
The simple answer to "What is the pH of salt?" is: it depends. Pure salt, specifically sodium chloride (NaCl), dissolved in pure water has a neutral pH of 7. However, the pH of a saline solution can vary depending on several factors, making this seemingly straightforward question surprisingly complex. This article delves into the intricacies of salt's pH, exploring the science behind it and the factors that influence its measurement.
Understanding pH and the pH Scale
Before we dive into the pH of salt, let's refresh our understanding of pH. The pH scale is a logarithmic scale that measures the acidity or alkalinity of a solution. It ranges from 0 to 14, with:
- pH 7: Neutral – indicating equal concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻).
- pH < 7: Acidic – indicating a higher concentration of hydrogen ions.
- pH > 7: Alkaline (or basic) – indicating a higher concentration of hydroxide ions.
Each whole number change on the pH scale represents a tenfold change in the concentration of hydrogen ions. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
The pH of Pure Sodium Chloride Solution
When pure sodium chloride (table salt) is dissolved in pure water, it undergoes dissociation:
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
Neither sodium ions (Na⁺) nor chloride ions (Cl⁻) react significantly with water to produce H⁺ or OH⁻ ions. Therefore, the concentration of H⁺ and OH⁻ ions remains essentially equal, resulting in a neutral pH of 7. This is true for ideal conditions, using distilled or deionized water.
Factors Affecting the pH of Salt Solutions
While a solution of pure NaCl in pure water will have a neutral pH, several factors can alter the pH of a saline solution:
1. Impurities in the Salt
Commercial table salt is not pure NaCl. It often contains small amounts of other compounds, such as magnesium chloride (MgCl₂), calcium chloride (CaCl₂), and potassium chloride (KCl). These impurities can affect the pH of the solution. For instance, magnesium chloride and calcium chloride can slightly increase the acidity of the solution, while potassium chloride might have a minimal impact on pH. The extent of this effect depends on the concentration and nature of the impurities present.
2. Impurities in the Water
The quality of the water used to dissolve the salt significantly impacts the final pH. Using tap water instead of distilled or deionized water introduces various ions and minerals that can alter the pH. These impurities can act as buffers, resisting changes in pH or directly contribute to acidity or alkalinity. The mineral content of tap water varies significantly depending on geographical location and water treatment processes.
3. Carbon Dioxide (CO₂) Absorption
Water readily absorbs carbon dioxide (CO₂) from the atmosphere. CO₂ reacts with water to form carbonic acid (H₂CO₃), a weak acid:
CO₂(g) + H₂O(l) ⇌ H₂CO₃(aq)
This carbonic acid then partially dissociates, releasing hydrogen ions (H⁺) and lowering the pH of the water. Therefore, even when dissolving pure NaCl in initially neutral water, the absorption of CO₂ from the air can slightly lower the pH over time. This effect is more pronounced in solutions with lower ionic strength.
4. Hydrolysis of Salt from Weak Acids or Bases
Salts formed from the reaction of a weak acid and a strong base, or a strong acid and a weak base, can undergo hydrolysis. Hydrolysis is a reaction with water. This reaction can affect the pH of the resulting solution. For example, sodium acetate (CH₃COONa), the salt of a weak acid (acetic acid) and a strong base (sodium hydroxide), produces a slightly alkaline solution due to the hydrolysis of the acetate ion. Conversely, ammonium chloride (NH₄Cl), the salt of a strong acid (hydrochloric acid) and a weak base (ammonia), produces a slightly acidic solution due to the hydrolysis of the ammonium ion. Sodium chloride, being the salt of a strong acid (hydrochloric acid) and a strong base (sodium hydroxide), does not undergo significant hydrolysis and maintains a neutral pH.
5. Temperature
Temperature can also influence the pH of a saline solution. The solubility of salts, and consequently the ion concentrations, changes with temperature. These changes in ion concentration can subtly affect the pH. The effect is usually small, but measurable, particularly at higher temperatures.
6. Concentration of the Salt Solution
The concentration of the salt solution itself can influence pH measurements, especially when dealing with highly concentrated solutions. The activity of ions deviates from their concentration at high concentrations, affecting the equilibrium between H⁺ and OH⁻ ions. This effect is usually only significant at very high salt concentrations.
Measuring the pH of Salt Solutions
The pH of a salt solution can be measured using a pH meter or pH indicator strips. A pH meter provides a more accurate and precise measurement, while indicator strips offer a quicker, less precise estimation. For accurate measurements, it is crucial to calibrate the pH meter before use and ensure the electrode is properly cleaned and maintained. The temperature of the solution should also be considered, as it can influence the accuracy of the reading.
Practical Applications and Implications
Understanding the pH of salt solutions is vital in various applications:
-
Aquaculture: Maintaining the correct pH in saltwater aquariums is crucial for the health and survival of aquatic organisms. The pH of the saltwater used in aquariums needs careful monitoring and adjustment as necessary. Inappropriate pH levels can stress or even kill the organisms.
-
Food Processing: Salt is used extensively in food processing, affecting the pH of the final product. This is particularly important in preserving foods, where specific pH ranges are necessary to inhibit microbial growth.
-
Chemical Industries: Many chemical processes involve saline solutions, and controlling the pH is often critical for reaction efficiency and product quality. Deviation from the desired pH can impact reaction rates, yield, and even product safety.
-
Medicine: Saline solutions are commonly used in medicine, particularly in intravenous fluids. Strict pH control is essential to prevent adverse effects on patients. Any deviation from the physiological pH range can have serious health consequences.
-
Environmental Science: The pH of natural water bodies can be affected by salinity, influencing the aquatic ecosystem. Understanding this relationship is important for managing water resources and protecting the environment.
Conclusion
While pure sodium chloride dissolved in pure water exhibits a neutral pH of 7, the pH of a saline solution is not always straightforward. Various factors, including impurities in the salt and water, CO₂ absorption, hydrolysis, temperature, and concentration, can influence the final pH. Accurate measurement requires proper technique and consideration of these factors. Understanding the factors that affect the pH of saline solutions is crucial in many scientific, industrial, and environmental applications, impacting everything from aquaculture to medicine. Precise control and monitoring of pH in saline solutions are often essential for ensuring optimal outcomes and preventing adverse effects.
Latest Posts
Latest Posts
-
Definition Of Line In A Poem
Mar 31, 2025
-
Speed Of A Wave On A String
Mar 31, 2025
-
Is There Water In The Desert
Mar 31, 2025
-
Definition Of Marginal Analysis In Economics
Mar 31, 2025
-
How To Describe Distribution Of Data
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph Of Salt . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
