Activity Series Of Metals And Non Metals
Muz Play
Mar 28, 2025 · 6 min read
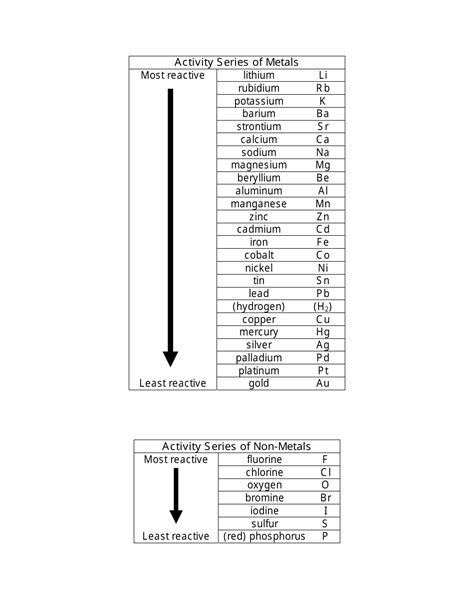
Table of Contents
The Activity Series of Metals and Nonmetals: A Comprehensive Guide
The activity series, also known as the reactivity series, is a crucial concept in chemistry that dictates how readily elements, particularly metals and nonmetals, undergo chemical reactions. Understanding this series is essential for predicting the outcome of various chemical processes, from simple displacement reactions to the complexities of electrochemical cells. This comprehensive guide will delve deep into the activity series of both metals and nonmetals, exploring its implications and applications.
Understanding the Activity Series of Metals
The activity series of metals arranges metals in order of their decreasing reactivity. The most reactive metals are located at the top, while the least reactive (or noble) metals are at the bottom. This reactivity is primarily determined by the ease with which a metal atom loses its valence electrons to form positive ions. Metals higher on the series readily lose electrons and readily participate in reactions, while those lower down are less inclined to do so.
Key Observations from the Metal Activity Series:
-
Displacement Reactions: A more reactive metal can displace a less reactive metal from its compound. For instance, if you place a piece of zinc metal (Zn) into a solution of copper(II) sulfate (CuSO₄), the zinc will displace the copper, forming zinc sulfate (ZnSO₄) and elemental copper (Cu). This is because zinc is higher on the activity series than copper. The reaction can be represented as:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq) + Cu(s) -
Reaction with Acids: Highly reactive metals, such as alkali metals (Group 1) and alkaline earth metals (Group 2), readily react with dilute acids like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄), producing hydrogen gas (H₂) and a metal salt. Less reactive metals may react only with concentrated acids or not at all.
-
Reaction with Water: The most reactive metals, like sodium (Na) and potassium (K), react vigorously with cold water, producing hydrogen gas and a metal hydroxide. Others, like magnesium (Mg), react more slowly, while some metals, like copper (Cu), do not react with water at all under normal conditions.
-
Oxidation and Reduction: The activity series directly relates to oxidation and reduction processes. A metal higher on the series is more easily oxidized (loses electrons), while a metal lower on the series is more easily reduced (gains electrons). This forms the basis of many electrochemical processes.
A Typical Metal Activity Series (from most to least reactive):
Lithium (Li) > Potassium (K) > Calcium (Ca) > Sodium (Na) > Magnesium (Mg) > Aluminum (Al) > Zinc (Zn) > Iron (Fe) > Nickel (Ni) > Tin (Sn) > Lead (Pb) > Hydrogen (H) > Copper (Cu) > Mercury (Hg) > Silver (Ag) > Platinum (Pt) > Gold (Au)
Note: The exact order might vary slightly depending on the reaction conditions and the specific reference source. Hydrogen is included as a reference point to compare the reactivity of metals with acids.
Applications of the Metal Activity Series:
The metal activity series has numerous practical applications, including:
-
Predicting Reaction Outcomes: Chemists use it to predict whether a reaction between a metal and a compound containing another metal will occur.
-
Extraction of Metals: The activity series guides the selection of appropriate methods for extracting metals from their ores. Highly reactive metals require more energy-intensive methods like electrolysis, while less reactive metals can be extracted through simpler techniques like reduction with carbon.
-
Corrosion Protection: Understanding the reactivity of metals helps in designing corrosion protection strategies. For instance, galvanization (coating iron with zinc) works because zinc is more reactive and will preferentially corrode, protecting the iron beneath.
-
Electrochemistry: The activity series is fundamental to understanding electrochemical cells, like batteries. The voltage generated by a battery depends on the difference in reactivity between the two metals used in the cell.
Understanding the Activity Series of Nonmetals
The activity series for nonmetals is less straightforward than that for metals. While metals tend to lose electrons, nonmetals tend to gain electrons. Therefore, the activity series for nonmetals reflects their relative tendency to gain electrons and form negative ions. The most reactive nonmetals are at the top, and the least reactive are at the bottom.
Key Considerations for the Nonmetal Activity Series:
-
Electron Affinity: The activity of a nonmetal is strongly related to its electron affinity – the energy change that occurs when an atom gains an electron. Nonmetals with high electron affinity readily accept electrons and are more reactive.
-
Electronegativity: Electronegativity, the tendency of an atom to attract electrons in a chemical bond, is another crucial factor influencing the reactivity of nonmetals. Highly electronegative nonmetals are more reactive.
-
Reactions with Metals: More reactive nonmetals can displace less reactive nonmetals from their compounds. For example, chlorine (Cl₂) can displace iodine (I₂) from potassium iodide (KI):
Cl₂(g) + 2KI(aq) → 2KCl(aq) + I₂(s) -
Reactions with Hydrogen: Many nonmetals react with hydrogen to form covalent hydrides. The reactivity in these reactions often reflects the nonmetal's position on the activity series.
A Typical Nonmetal Activity Series (from most to least reactive):
Fluorine (F) > Chlorine (Cl) > Bromine (Br) > Iodine (I) > Sulfur (S) > Phosphorus (P) > Carbon (C)
Note: This series is not as universally agreed upon as the metal activity series and can vary depending on the specific reaction conditions and the type of reaction being considered. The order shown reflects a general trend based on common reactions.
Applications of the Nonmetal Activity Series:
The nonmetal activity series has important applications in various fields:
-
Predicting Reactions: Just as with metals, the nonmetal activity series helps in predicting the outcome of reactions between nonmetals and their compounds.
-
Industrial Processes: This series is crucial in understanding and optimizing various industrial processes involving nonmetals, such as the production of halogens and other chemicals.
-
Environmental Chemistry: Understanding the reactivity of nonmetals is essential for studying environmental processes, such as the formation and breakdown of pollutants.
-
Material Science: The activity series guides the development and selection of materials with specific properties, based on the reactivity of nonmetals incorporated into them.
Comparing Metal and Nonmetal Activity Series:
While both series describe relative reactivity, some key differences exist:
-
Electron Transfer: Metals lose electrons, while nonmetals gain electrons in chemical reactions.
-
Ion Formation: Metals form positive ions (cations), while nonmetals form negative ions (anions).
-
Types of Reactions: Metals are primarily involved in displacement reactions and reactions with acids and water. Nonmetals participate in reactions with metals and hydrogen, as well as displacement reactions between nonmetals themselves.
-
Series Consistency: The metal activity series is more consistently defined and widely accepted compared to the nonmetal activity series, which can show more variations based on specific reactions.
Conclusion:
The activity series of metals and nonmetals are powerful tools for predicting and understanding chemical reactions. Understanding these series is essential for various applications, from industrial processes to environmental studies. While the metal activity series offers a more established and consistent framework, the nonmetal activity series still provides valuable insights into the relative reactivity of these elements. Mastering these concepts is crucial for success in chemistry at all levels. Further research and exploration into specific reactions and their mechanisms can provide a deeper understanding of these crucial concepts within the broader field of chemistry. Remember that while these series provide excellent predictive power, careful consideration of specific reaction conditions is always necessary for accurate predictions.
Latest Posts
Latest Posts
-
Using The Small X Approximation To Solve Equilibrium Problems
Mar 31, 2025
-
What Are Group 17 Elements Called
Mar 31, 2025
-
What Are Lone Pair Of Electrons
Mar 31, 2025
-
Definition Of Line In A Poem
Mar 31, 2025
-
Speed Of A Wave On A String
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Activity Series Of Metals And Non Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
