What Are Group 17 Elements Called
Muz Play
Mar 31, 2025 · 7 min read
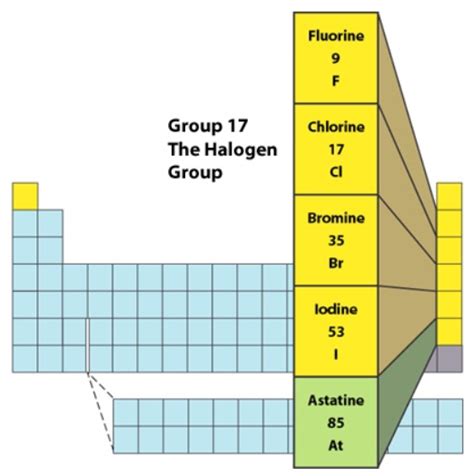
Table of Contents
What Are Group 17 Elements Called? Exploring the Halogens
Group 17 elements, also known as halogens, are a fascinating group of nonmetals with unique properties that make them essential in various applications, from everyday life to cutting-edge technologies. Understanding their characteristics, reactivity, and uses is crucial to appreciating their significance in the world around us. This comprehensive article delves into the world of halogens, explaining their naming, properties, reactions, and applications in detail.
Understanding the Name: Halogens
The term "halogen" originates from the Greek words "hals" (meaning "salt") and "genes" (meaning "forming"). This perfectly encapsulates the key characteristic of these elements: their ability to react with metals to form salts. Think of common table salt, sodium chloride (NaCl); the chlorine in this compound is a classic example of a halogen's salt-forming prowess. This salt-forming tendency is a defining feature that sets them apart from other groups in the periodic table.
More than Just Salt Formation
While salt formation is a crucial defining factor, it's important to understand that the halogens' reactivity extends far beyond just metals. They readily react with a wide range of elements and compounds, demonstrating a remarkable versatility in their chemical behavior. Their high electronegativity, a measure of an atom's ability to attract electrons in a chemical bond, is the driving force behind this reactivity. This tendency to gain electrons to achieve a stable electron configuration is what makes them such powerful oxidizing agents.
The Halogen Family: A Closer Look at the Members
The halogen family consists of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Each element exhibits similar chemical properties due to their shared electron configuration, but their physical properties vary significantly due to differences in atomic size and intermolecular forces.
Fluorine (F): The Most Reactive
Fluorine, the lightest halogen, is notoriously reactive. Its small atomic size and high electronegativity make it the most electronegative element on the periodic table. This extreme reactivity translates to a highly oxidizing nature, capable of reacting violently with many substances, including water. Pure fluorine is incredibly dangerous to handle and requires specialized equipment and precautions. Despite its inherent dangers, fluorine plays a vital role in various applications, including:
- Fluoridation of water: A small amount of fluoride is added to drinking water to help prevent tooth decay.
- Teflon production: Polytetrafluoroethylene (PTFE), commonly known as Teflon, is a non-stick coating used in cookware and other applications.
- Refrigerants: While CFCs (chlorofluorocarbons) are being phased out due to their ozone-depleting properties, some fluorinated compounds are still used as refrigerants.
Chlorine (Cl): A Versatile Element
Chlorine, a greenish-yellow gas, is significantly less reactive than fluorine but still highly reactive. Its most well-known application is in water purification, where it effectively kills harmful bacteria and other microorganisms. Beyond water treatment, chlorine finds applications in:
- Bleach production: Chlorine is a key component in many household bleaches, used for disinfecting and whitening.
- PVC production: Polyvinyl chloride (PVC) is a versatile plastic used in pipes, flooring, and other applications.
- Industrial chemicals: Chlorine is a crucial building block for many other industrial chemicals.
Bromine (Br): The Only Liquid Nonmetal
Bromine is unique among the halogens as it's the only one that exists as a liquid at room temperature. Its reddish-brown color and pungent odor make it easily recognizable. Bromine is less reactive than chlorine and fluorine but still exhibits significant reactivity. Its applications include:
- Flame retardants: Brominated flame retardants are used in various materials to prevent fires. (Note: Environmental concerns surrounding some brominated flame retardants have led to restrictions and replacements in many applications.)
- Agricultural chemicals: Certain bromine-containing compounds are used as pesticides and fumigants. (Again, environmental concerns are leading to more sustainable alternatives.)
- Medical applications: Bromine compounds have been used in some medical applications, although their use is decreasing due to safety concerns.
Iodine (I): Essential for Human Health
Iodine, a shiny, dark-gray solid, is crucial for human health. It's a component of thyroid hormones, which regulate metabolism. Iodine deficiency can lead to various health problems, including goiter. Its applications include:
- Dietary supplements: Iodine is added to table salt and other foods to prevent iodine deficiency.
- Disinfectants: Iodine is used in various antiseptic solutions and disinfectants.
- X-ray contrast agents: Iodine compounds are used in X-ray contrast agents to help visualize organs and tissues.
Astatine (At): The Radioactive Halogen
Astatine, the heaviest halogen, is a radioactive element with a very short half-life. Because of its radioactivity, it's extremely rare and difficult to study. It's not widely used in any practical applications due to its instability.
Chemical Reactions of Halogens: A Deeper Dive
The halogens' high electronegativity leads to several key reaction patterns:
Reaction with Metals: Salt Formation
As previously mentioned, halogens readily react with metals to form ionic salts. For example, the reaction of sodium (Na) with chlorine (Cl) produces sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
This reaction is a classic example of an oxidation-reduction (redox) reaction, where sodium loses electrons (oxidation) and chlorine gains electrons (reduction).
Reaction with Nonmetals: Covalent Compounds
Halogens also react with nonmetals to form covalent compounds. These compounds are characterized by the sharing of electrons between atoms. For example, the reaction of chlorine with hydrogen produces hydrogen chloride (HCl), a strong acid:
H₂(g) + Cl₂(g) → 2HCl(g)
Displacement Reactions: Halogen Reactivity Series
Halogens exhibit a clear reactivity trend, with fluorine being the most reactive and astatine the least. This reactivity is reflected in displacement reactions, where a more reactive halogen can displace a less reactive halogen from its compounds. For example, chlorine can displace bromine from potassium bromide:
Cl₂(g) + 2KBr(aq) → 2KCl(aq) + Br₂(l)
This reaction highlights the relative oxidizing power of the halogens.
Applications of Halogens: A Wide Range of Uses
The halogens' diverse properties have led to their widespread use across various industries:
Industrial Applications
- Plastics and polymers: Chlorine and fluorine are crucial components in many plastics and polymers, including PVC, Teflon, and various fluoropolymers.
- Refrigerants: While CFCs are being phased out, some fluorinated compounds are still used as refrigerants, but with a focus on ozone-friendly alternatives.
- Pesticides and herbicides: Certain halogen-containing compounds are used as pesticides and herbicides, although their use is increasingly scrutinized due to environmental concerns.
Medical and Pharmaceutical Applications
- Disinfectants and antiseptics: Chlorine and iodine are widely used as disinfectants and antiseptics.
- Medical imaging: Iodine compounds are used in X-ray contrast agents.
- Thyroid hormone production: Iodine is essential for the production of thyroid hormones.
Other Applications
- Water treatment: Chlorine is a key component in water treatment processes to kill harmful bacteria and microorganisms.
- Bleaching agents: Chlorine is used in many household and industrial bleaching agents.
- Flame retardants: Bromine compounds have been used as flame retardants, although their use is being reevaluated due to environmental concerns.
Environmental Concerns and Safety Precautions
While halogens have numerous beneficial applications, some pose significant environmental and health risks:
- Ozone depletion: CFCs, previously widely used as refrigerants, were found to deplete the ozone layer, leading to international regulations to phase them out.
- Toxicity: Many halogen compounds are toxic and require careful handling and disposal.
- Persistence in the environment: Some halogenated compounds persist in the environment for long periods, potentially causing harm to ecosystems.
Therefore, responsible use, careful handling, and proper disposal are paramount when working with halogens and their compounds.
Conclusion: The Importance of Halogens
The halogens, with their remarkable reactivity and versatility, play a significant role in various aspects of our lives. From essential elements in human health to crucial components in industrial processes, their impact is undeniable. However, it's crucial to acknowledge and address the potential environmental and health risks associated with some halogen compounds to ensure their sustainable and responsible use. Continued research and development of safer alternatives will be key to maximizing the benefits while mitigating the risks associated with these fascinating elements. Further research into the applications and environmental impact of halogens is ongoing, ensuring a safer and more sustainable future. Understanding the properties and applications of halogens provides crucial insights into the complex interplay between chemistry and the world around us.
Latest Posts
Latest Posts
-
How To Place A Condom Catheter
Apr 02, 2025
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
-
Examples Of Liquid In Liquid Solution
Apr 02, 2025
-
Diagram Of Salt Dissolving In Water
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are Group 17 Elements Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
