What Are Lone Pair Of Electrons
Muz Play
Mar 31, 2025 · 6 min read
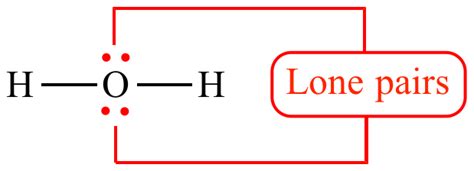
Table of Contents
What are Lone Pairs of Electrons? A Deep Dive into Valence Shell Electron Pairs
Understanding chemical bonding and molecular geometry requires a firm grasp of fundamental concepts, and among them, lone pairs of electrons hold a crucial position. These unshared electron pairs significantly influence a molecule's shape, polarity, and reactivity. This comprehensive guide will explore lone pairs in detail, explaining their nature, impact on molecular geometry, and their role in various chemical phenomena.
What are Lone Pairs?
Lone pairs, also known as non-bonding pairs or unshared pairs, are pairs of valence electrons that are not involved in covalent bonding. Unlike bonding pairs, which are shared between two atoms, lone pairs are associated solely with a single atom. They reside in the atom's valence shell, the outermost electron shell, and contribute significantly to the atom's overall electron configuration and properties.
Visualizing Lone Pairs
Imagine the valence shell of an atom as a space with specific regions where electrons are most likely to be found. Bonding pairs occupy specific regions between two atoms, forming a covalent bond. Lone pairs, however, occupy regions solely on a single atom, creating a distinct electron density around that atom. These regions are often depicted as "clouds" or "pairs of dots" in Lewis dot structures.
Where do Lone Pairs Come From?
Lone pairs originate from the valence electrons of an atom. The number of valence electrons an atom possesses determines its bonding capacity and the potential number of lone pairs it can have. For example, oxygen (O) has six valence electrons. When it forms two covalent bonds (e.g., in a water molecule, H₂O), two pairs of electrons are involved in bonding, leaving two pairs as lone pairs on the oxygen atom.
The Impact of Lone Pairs on Molecular Geometry
Lone pairs significantly influence the three-dimensional arrangement of atoms in a molecule – its molecular geometry. This influence stems from the fact that lone pairs exert a stronger repulsive force than bonding pairs. This repulsion forces the bonding pairs and the lone pairs to arrange themselves in a way that minimizes repulsion and maximizes the distance between them.
VSEPR Theory: The Guiding Principle
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a cornerstone in predicting molecular geometry. This theory posits that electron pairs (both bonding and lone pairs) repel each other and will arrange themselves to be as far apart as possible. The arrangement that minimizes repulsion determines the molecule's shape.
Examples of Lone Pair Influence:
-
Water (H₂O): Oxygen has two lone pairs and two bonding pairs. The ideal arrangement to minimize repulsion would be a tetrahedral arrangement (109.5° bond angles). However, due to the stronger repulsion of the lone pairs, the bond angle is compressed to approximately 104.5°. This results in a bent molecular geometry.
-
Ammonia (NH₃): Nitrogen has one lone pair and three bonding pairs. Similar to water, the ideal arrangement is tetrahedral. However, the lone pair's repulsion compresses the bond angles slightly, resulting in a trigonal pyramidal geometry.
-
Methane (CH₄): Carbon has four bonding pairs and no lone pairs. The four bonding pairs arrange themselves tetrahedrally with bond angles of 109.5°, resulting in a tetrahedral geometry.
The examples clearly demonstrate how the presence and number of lone pairs dramatically affect the final molecular geometry, deviating from the ideal angles predicted by the VSEPR theory.
Lone Pairs and Molecular Polarity
Lone pairs are crucial in determining a molecule's polarity. A molecule is considered polar if it possesses a net dipole moment, meaning there is an uneven distribution of electron density. Lone pairs contribute to this uneven distribution.
Understanding Dipole Moments
A dipole moment arises from the difference in electronegativity between atoms. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. When atoms with different electronegativities bond, the electron density is pulled towards the more electronegative atom, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom.
Lone Pairs and Dipole Moments:
Lone pairs contribute to a molecule's dipole moment because they create a region of high electron density on a single atom. This high electron density can significantly influence the overall dipole moment of the molecule. Even if the individual bonds are nonpolar (atoms have similar electronegativities), the presence of lone pairs can induce a net dipole moment.
Lone Pairs and Reactivity
Lone pairs play a critical role in the reactivity of molecules. They act as electron donors, participating in various chemical reactions.
Lewis Bases: The Electron Donors
Molecules with lone pairs are often classified as Lewis bases. A Lewis base is a species that can donate a pair of electrons to form a coordinate covalent bond (also known as a dative bond). This donation occurs when the lone pair interacts with a Lewis acid, a species that can accept a pair of electrons.
Examples of Lone Pair Reactivity:
-
Nucleophilic Attack: Lone pairs can participate in nucleophilic attacks. A nucleophile is a species that is attracted to positively charged or electron-deficient centers. The lone pair on the nucleophile attacks the electrophile (electron-deficient species), forming a new bond.
-
Coordination Chemistry: Lone pairs are essential in coordination chemistry. Transition metal complexes frequently involve ligands (molecules or ions) donating lone pairs to the metal center. These lone pair donations form coordinate bonds, leading to the formation of stable complexes.
-
Acid-Base Reactions: Lone pairs are involved in many acid-base reactions. For example, ammonia (NH₃), with its lone pair, acts as a Brønsted-Lowry base by accepting a proton (H⁺).
Advanced Concepts and Applications
The influence of lone pairs extends beyond the introductory concepts discussed above. Their role in more advanced chemical phenomena deserves further consideration.
Hypervalence: Challenging the Octet Rule
Some molecules, especially those containing elements from the third period and beyond, can accommodate more than eight valence electrons around a central atom. This phenomenon is known as hypervalence. Lone pairs often play a role in enabling hypervalence by expanding the valence shell to accommodate additional electrons. However, the role of lone pairs in hypervalence is still a subject of debate among chemists.
Molecular Orbital Theory: A More Detailed Perspective
While VSEPR theory provides a simplified model for predicting molecular geometry, Molecular Orbital (MO) theory offers a more sophisticated description. MO theory considers the combination of atomic orbitals to form molecular orbitals, which are regions of space where electrons are delocalized. Lone pairs are still present in MO theory, but they are described as electrons occupying non-bonding molecular orbitals.
Spectroscopy and Lone Pairs:
Different spectroscopic techniques, such as infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy, can provide experimental evidence for the presence and characteristics of lone pairs. Lone pairs influence the vibrational frequencies observed in IR spectroscopy and the chemical shifts in NMR spectroscopy.
Conclusion
Lone pairs of electrons are fundamental to understanding molecular structure, reactivity, and properties. From shaping molecular geometry and influencing polarity to acting as electron donors in chemical reactions, these unshared electron pairs play a multifaceted role in the fascinating world of chemistry. A comprehensive understanding of lone pairs provides a solid foundation for exploring more complex chemical concepts and phenomena. Further exploration into advanced topics, such as hypervalence and the application of spectroscopic techniques, will provide a deeper appreciation for the importance of lone pairs in the diverse field of chemistry. Their influence extends across various applications, impacting the design of new materials, pharmaceuticals, and other technologies, highlighting the significance of these seemingly simple electron pairs.
Latest Posts
Latest Posts
-
How To Write Quadratic Equation From Graph
Apr 02, 2025
-
How To Place A Condom Catheter
Apr 02, 2025
-
How Many Elements Are Gases At Room Temperature
Apr 02, 2025
-
3 Main Ideas Of Cell Theory
Apr 02, 2025
-
Examples Of Liquid In Liquid Solution
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Are Lone Pair Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
