How Many Unpaired Electrons Does Carbon Have
Muz Play
Mar 28, 2025 · 5 min read
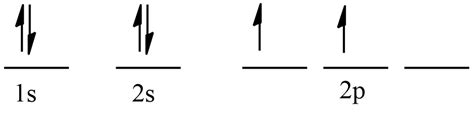
Table of Contents
How Many Unpaired Electrons Does Carbon Have? A Deep Dive into Carbon's Electronic Structure
Carbon, the cornerstone of organic chemistry and the building block of life as we know it, possesses a fascinating electronic structure that dictates its remarkable versatility. Understanding the number of unpaired electrons in a carbon atom is crucial to grasping its bonding behavior and the vast array of molecules it forms. This article delves into the intricacies of carbon's electron configuration, explaining how many unpaired electrons it possesses in its ground state and excited states, and exploring the implications for its chemical reactivity.
Carbon's Electronic Configuration: The Foundation
To determine the number of unpaired electrons, we need to examine carbon's electronic configuration. Carbon (C) has an atomic number of 6, meaning it has six protons and, in a neutral atom, six electrons. These electrons are distributed among different energy levels and orbitals according to the Aufbau principle and Hund's rule.
The electronic configuration of carbon in its ground state is 1s²2s²2p². Let's break this down:
- 1s²: Two electrons occupy the 1s orbital, the lowest energy level. These electrons are paired, meaning they have opposite spins.
- 2s²: Two electrons fill the 2s orbital, the next higher energy level. Again, these electrons are paired.
- 2p²: Two electrons occupy the 2p subshell, which consists of three 2p orbitals (2px, 2py, and 2pz). Crucially, according to Hund's rule, these two electrons will occupy separate 2p orbitals with parallel spins before pairing up in the same orbital.
Therefore, in its ground state, carbon has two unpaired electrons. These unpaired electrons reside in separate 2p orbitals, making carbon readily available to form covalent bonds. This is the key to carbon's ability to form a vast number of stable compounds.
Hund's Rule: The Key to Unpaired Electrons
Hund's rule of maximum multiplicity dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This is because electrons, being negatively charged, repel each other. Occupying separate orbitals minimizes this repulsion, leading to a lower overall energy state and greater stability. This principle is vital in understanding why carbon has unpaired electrons in its ground state.
Implications of Unpaired Electrons: Carbon's Bonding Prowess
The presence of two unpaired electrons in carbon's ground state directly relates to its tetravalency – its ability to form four covalent bonds. Each unpaired electron can participate in a covalent bond with another atom, sharing its electron to achieve a stable octet configuration (eight electrons in its outermost shell). This explains the vast array of organic molecules possible, from simple methane (CH₄) to complex proteins and DNA.
Hybridization: Expanding Carbon's Bonding Capabilities
While the ground state configuration explains the basic bonding, carbon's ability to form more complex structures involves hybridization. This process involves mixing atomic orbitals to create new hybrid orbitals with different shapes and energies, which are more effective at forming bonds. The most common hybridization in carbon is sp³, sp², and sp.
-
sp³ hybridization: In this case, one 2s and three 2p orbitals hybridize to form four equivalent sp³ hybrid orbitals, each with one unpaired electron. This allows carbon to form four single bonds, as seen in methane (CH₄).
-
sp² hybridization: One 2s and two 2p orbitals hybridize to form three sp² hybrid orbitals, each with one unpaired electron. The remaining 2p orbital remains unhybridized. This configuration enables carbon to form three sigma bonds and one pi bond, as seen in ethene (C₂H₄).
-
sp hybridization: One 2s and one 2p orbital hybridize to form two sp hybrid orbitals, each with one unpaired electron. The two remaining 2p orbitals are unhybridized. This leads to the formation of two sigma bonds and two pi bonds, as seen in ethyne (C₂H₂).
In each of these hybridization scenarios, the number of unpaired electrons directly relates to the number of bonds the carbon atom can form. The unpaired electrons are crucial in forming the covalent bonds that hold molecules together.
Excited States and Unpaired Electrons
While the ground state configuration is the most stable, carbon can be excited by absorbing energy. This energy can promote an electron from a lower energy level to a higher energy level, potentially changing the number of unpaired electrons.
For instance, if an electron is promoted from the 2s orbital to a vacant 2p orbital, the excited state configuration becomes 1s²2s¹2p³. Now, carbon would have four unpaired electrons. This excited state is less stable than the ground state but plays a significant role in some chemical reactions. The additional unpaired electrons increase the reactivity of the carbon atom.
Carbon's Role in Organic Chemistry and Biochemistry
The unique electronic structure of carbon, specifically its tendency to have unpaired electrons readily available for bonding, is the foundation of organic chemistry and biochemistry. The vast diversity of organic molecules stems directly from carbon's ability to form strong, stable covalent bonds with itself and other elements like hydrogen, oxygen, nitrogen, and sulfur. This ability to form long chains, branched structures, and rings is unmatched by any other element.
This tetravalency, resulting from its unpaired electrons, enables the creation of an immense array of molecules with diverse properties and functionalities. From simple hydrocarbons to complex biomolecules like proteins, carbohydrates, and nucleic acids, carbon is the essential element underpinning the complexity of life. Understanding how its unpaired electrons facilitate bonding is fundamental to understanding the structures and functions of these biomolecules.
Carbon's Abundance and its Significance for Life
Carbon's abundance in the universe and its unique chemical properties make it indispensable for life as we know it. The ability of carbon atoms to bond extensively with each other and with other elements allows for the formation of an incredible variety of complex molecules that serve as the building blocks of life.
Conclusion: The Significance of Unpaired Electrons
In summary, carbon in its ground state possesses two unpaired electrons. This seemingly simple fact is profoundly significant. These unpaired electrons are responsible for carbon's tetravalency, its ability to form four covalent bonds, leading to the incredible diversity and complexity of organic and biological molecules. The understanding of these unpaired electrons and their role in hybridization is fundamental to comprehending the vast realm of organic chemistry and biochemistry, and ultimately, the very basis of life itself. Further exploration into excited states and their impact on reactivity enhances this fundamental understanding even more, showcasing the truly remarkable nature of this ubiquitous element.
Latest Posts
Latest Posts
-
Using The Small X Approximation To Solve Equilibrium Problems
Mar 31, 2025
-
What Are Group 17 Elements Called
Mar 31, 2025
-
What Are Lone Pair Of Electrons
Mar 31, 2025
-
Definition Of Line In A Poem
Mar 31, 2025
-
Speed Of A Wave On A String
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
