Where Does Oxidation Occur In A Voltaic Cell
Muz Play
Mar 20, 2025 · 6 min read
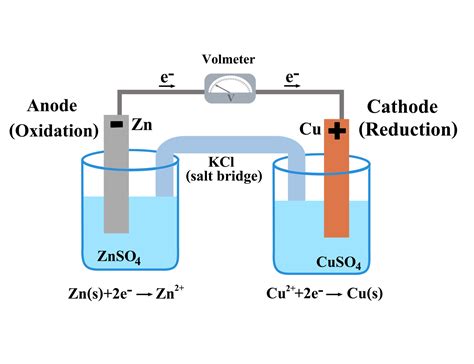
Table of Contents
Where Does Oxidation Occur in a Voltaic Cell? Understanding Redox Reactions
Voltaic cells, also known as galvanic cells, are electrochemical cells that convert chemical energy into electrical energy. This conversion relies on a fundamental chemical process: redox reactions, which involve the transfer of electrons between different species. Understanding where oxidation and reduction occur within a voltaic cell is crucial to grasping how these cells function. This article will delve deep into the specifics of oxidation within a voltaic cell, exploring its location, the role of the anode, and the broader context of redox reactions.
The Fundamentals of Redox Reactions
Before we pinpoint the location of oxidation in a voltaic cell, let's review the basics of redox reactions. These reactions are always coupled, meaning oxidation and reduction occur simultaneously.
-
Oxidation: This process involves the loss of electrons. A species undergoing oxidation is called the reducing agent because it donates electrons, causing another species to be reduced. Remember the mnemonic OIL RIG – Oxidation Is Loss, Reduction Is Gain (of electrons).
-
Reduction: This process involves the gain of electrons. A species undergoing reduction is called the oxidizing agent because it accepts electrons, causing another species to be oxidized.
In a redox reaction, the number of electrons lost during oxidation must equal the number of electrons gained during reduction. This ensures overall charge balance.
The Anatomy of a Voltaic Cell: Identifying the Anode
A voltaic cell consists of two half-cells:
-
Anode: This is the electrode where oxidation takes place. It's the site where electrons are released. The anode is negatively charged because it's losing electrons.
-
Cathode: This is the electrode where reduction takes place. It's the site where electrons are accepted. The cathode is positively charged because it's gaining electrons.
The two half-cells are connected by a salt bridge or a porous membrane, which allows the flow of ions to maintain electrical neutrality in the cell. Electrons flow through an external circuit, connecting the anode and the cathode. This flow of electrons creates an electric current.
Therefore, to answer the central question: Oxidation occurs at the anode of a voltaic cell.
Detailed Look at Anode Oxidation: Examples and Mechanisms
Let's consider some specific examples to illustrate oxidation at the anode:
Example 1: The Zinc-Copper Voltaic Cell
A classic example is the zinc-copper voltaic cell. Here, a zinc electrode (Zn) is immersed in a zinc sulfate (ZnSO₄) solution, and a copper electrode (Cu) is immersed in a copper sulfate (CuSO₄) solution.
The oxidation half-reaction at the zinc anode is:
Zn(s) → Zn²⁺(aq) + 2e⁻
Zinc atoms lose two electrons, becoming zinc ions (Zn²⁺), which enter the solution. These electrons flow through the external circuit to the copper cathode.
Example 2: The Daniell Cell
The Daniell cell, similar in principle, uses zinc and copper electrodes. The oxidation half-reaction remains the same as in the previous example. The detailed mechanism involves the zinc atoms losing electrons to become zinc ions, leaving behind the electrons at the anode's surface.
Example 3: Involving Different Metals
The principle remains consistent even when different metals are used. For instance, a voltaic cell could be constructed with a magnesium anode and a silver cathode. Magnesium would undergo oxidation, losing electrons to become Mg²⁺ ions.
Mg(s) → Mg²⁺(aq) + 2e⁻
The electrons would then flow to the silver cathode, where silver ions (Ag⁺) would be reduced to silver metal.
Example 4: Non-Metallic Anodes
While the examples above use metallic anodes, it's crucial to understand that oxidation can also occur at non-metallic anodes. For instance, in some fuel cells, oxidation might involve the oxidation of hydrogen gas at a platinum anode.
2H₂(g) → 4H⁺(aq) + 4e⁻
This highlights the versatility of the oxidation process at the anode. The key is the loss of electrons, regardless of the chemical nature of the species involved.
Factors Affecting Oxidation at the Anode
Several factors influence the rate and extent of oxidation at the anode:
-
Standard Reduction Potential: The standard reduction potential (E°) of the anode material is a measure of its tendency to undergo reduction. A lower standard reduction potential indicates a greater tendency for the material to undergo oxidation.
-
Concentration: The concentration of the ions in solution affects the equilibrium of the redox reaction. Higher concentrations of the oxidized species can inhibit oxidation.
-
Temperature: Temperature influences the rate of the reaction. Higher temperatures generally increase the reaction rate.
-
Surface Area: A larger surface area of the anode provides more sites for oxidation to occur, increasing the rate of the reaction.
The Importance of the Salt Bridge
The salt bridge is essential for the continued functioning of the voltaic cell. It allows the flow of ions to maintain charge neutrality in the half-cells. Without it, a buildup of positive charge at the anode and negative charge at the cathode would quickly stop the electron flow. The ions migrating through the salt bridge help to balance these charges, preventing the cell from becoming electrically polarized and ensuring the continued oxidation at the anode and reduction at the cathode.
Applications of Voltaic Cells and Oxidation at the Anode
Voltaic cells have numerous applications, including:
-
Batteries: These portable power sources rely on redox reactions in voltaic cells to generate electricity. The oxidation at the anode is a key component of their operation.
-
Fuel Cells: These devices convert the chemical energy of a fuel (like hydrogen) into electricity through oxidation at the anode.
-
Corrosion Prevention: Understanding oxidation at the anode is crucial in preventing corrosion of metals. Sacrificial anodes are often used, where a more easily oxidized metal is used as the anode, protecting a more valuable metal from corrosion.
-
Electroplating: Electroplating involves using a voltaic cell to deposit a thin layer of metal onto a surface. The oxidation at the anode provides the metal ions that are reduced at the cathode to form the plating.
Conclusion: Oxidation – The Driving Force of Voltaic Cells
Oxidation at the anode is the cornerstone of voltaic cell operation. It's the process that initiates the electron flow, generating the electric current. Understanding the intricacies of oxidation, the factors affecting it, and its role within the broader context of redox reactions is fundamental to comprehending the function and applications of these crucial electrochemical devices. From batteries powering our everyday devices to the intricate workings of fuel cells, the process of oxidation at the anode remains a critical aspect of electrochemical energy conversion. Further research into materials science and electrochemistry continues to refine our understanding and improve the efficiency and applications of voltaic cells.
Latest Posts
Latest Posts
-
Chemiosmosis Atp Synthesis In Chloroplasts Answer Key
Mar 20, 2025
-
How To Find Expected Frequency From Observed Frequency
Mar 20, 2025
-
Confidence Interval For Population Means Calculator
Mar 20, 2025
-
The Statement That Growth Slows Down During Middle Childhood
Mar 20, 2025
-
Are Fatty Acids Polar Or Nonpolar
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Where Does Oxidation Occur In A Voltaic Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
