Kirby Bauer Method Of Antibiotic Sensitivity Testing
Muz Play
Mar 20, 2025 · 6 min read
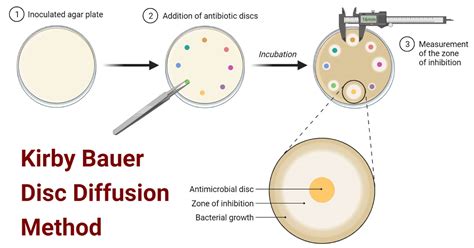
Table of Contents
Kirby-Bauer Method: A Comprehensive Guide to Antibiotic Sensitivity Testing
The Kirby-Bauer method, also known as the disk diffusion test, is a widely used laboratory technique for determining the susceptibility of bacteria to various antimicrobial agents. This method is crucial in guiding clinical decisions regarding antibiotic treatment, informing infection control strategies, and contributing to epidemiological surveillance of antibiotic resistance. Understanding its principles, procedure, interpretation, and limitations is essential for anyone involved in microbiology or infectious disease management.
Understanding the Principles of the Kirby-Bauer Method
The Kirby-Bauer test relies on the principle of diffusion. Antibiotic disks containing a known concentration of the antimicrobial agent are placed onto an agar plate that has been evenly inoculated with a standardized bacterial suspension. The antibiotics then diffuse from the disk into the surrounding agar, creating a concentration gradient. If the bacteria are susceptible to the antibiotic, a zone of inhibition (a clear area around the disk where bacterial growth is inhibited) will form. The size of this zone is directly related to the antibiotic's effectiveness against the bacteria. Larger zones indicate greater susceptibility, while smaller or absent zones suggest resistance.
Key Factors Influencing Zone of Inhibition
Several factors significantly influence the size of the zone of inhibition and, consequently, the interpretation of the results. These include:
- Antibiotic concentration: Disks contain a precise concentration of the antibiotic, which is standardized to ensure consistent results.
- Diffusion rate: The antibiotic's physicochemical properties influence its rate of diffusion through the agar. Some antibiotics diffuse faster than others.
- Bacterial inoculum size: A standardized bacterial suspension is crucial for reliable results. Too many bacteria will obscure the zone of inhibition, while too few may lead to falsely large zones.
- Incubation time and temperature: Optimal incubation conditions (typically 35°C for 16-18 hours) are essential for accurate bacterial growth and antibiotic diffusion.
- Agar depth: The depth of the agar must be standardized (typically 4 mm) to ensure consistent diffusion.
- Type of agar: Mueller-Hinton agar is the standard medium due to its consistent composition and lack of interfering substances.
Step-by-Step Procedure of the Kirby-Bauer Method
The Kirby-Bauer test involves several meticulously executed steps:
1. Preparation of Bacterial Suspension
A pure bacterial culture is essential. A standardized inoculum is prepared by adjusting the turbidity of a bacterial suspension to match a 0.5 McFarland standard. This ensures a consistent number of bacterial cells are present on the agar plate.
2. Inoculation of the Agar Plate
Using a sterile cotton swab, the bacterial suspension is evenly spread across the surface of a Mueller-Hinton agar plate. This ensures uniform bacterial growth across the plate. Excess inoculum should be removed by rotating the swab against the side of the tube.
3. Application of Antibiotic Disks
Sterile forceps are used to place antibiotic disks onto the inoculated agar plate. Each disk contains a specific antibiotic at a known concentration. The disks are spaced evenly apart to prevent overlapping zones of inhibition.
4. Incubation
The inoculated plate is incubated at a controlled temperature (typically 35°C) for 16-18 hours in an inverted position. This prevents condensation from accumulating on the agar surface, which could interfere with the results.
5. Measurement of Zones of Inhibition
After incubation, the zones of inhibition are carefully measured using a ruler in millimeters. The diameter of the zone is measured from the edge of the antibiotic disk to the edge of the clear area of inhibition. Any hazy or indistinct zones should be carefully assessed.
Interpretation of Results and Clinical Significance
The measurement of the zone of inhibition is crucial for interpreting the susceptibility of the bacteria to the antibiotic. Each antibiotic has established interpretive standards that define the size of the zone of inhibition corresponding to susceptible, intermediate, and resistant categories. These interpretive standards are provided by organizations like the Clinical and Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). These standards are essential for accurate interpretation and should always be consulted.
Susceptible: Bacteria exhibiting a zone of inhibition exceeding the established breakpoint are considered susceptible to the antibiotic. This indicates that the antibiotic is likely to be effective in treating the infection.
Intermediate: Bacteria with zone sizes falling within the intermediate range may respond to high concentrations of the antibiotic or prolonged therapy. Further testing may be needed to clarify the clinical implications.
Resistant: Bacteria showing zone sizes below the breakpoint are considered resistant to the antibiotic. This indicates that the antibiotic is unlikely to be effective in treating the infection, and alternative treatment options should be considered.
The Kirby-Bauer results are crucial for guiding antibiotic therapy. Choosing the right antibiotic based on susceptibility testing ensures optimal treatment, minimizes the risk of treatment failure, and reduces the selection pressure that drives antibiotic resistance.
Limitations of the Kirby-Bauer Method
Despite its widespread use, the Kirby-Bauer method has some limitations:
- Limited accuracy for certain antibiotics: Some antibiotics don't diffuse well in agar or are affected by other factors, limiting the accuracy of the method for these agents.
- Inability to detect low-level resistance: The method may not always detect low-level resistance, which could lead to treatment failure despite an apparently susceptible result.
- Lack of information on minimum inhibitory concentration (MIC): The Kirby-Bauer method only provides qualitative information about susceptibility (susceptible, intermediate, resistant). It does not provide the MIC, which is the lowest concentration of an antibiotic that inhibits bacterial growth. Determining MIC requires more sophisticated techniques like broth dilution methods.
- Time-consuming: The method requires 16-18 hours of incubation, which can delay treatment decisions.
- Requires skilled personnel: Accurate execution and interpretation of the test require experienced laboratory personnel.
Quality Control and Standardization
Strict adherence to standardized protocols is crucial for obtaining reliable results. Quality control measures involve using standardized bacterial strains with known susceptibilities to ensure the accuracy of the test. These control strains are tested alongside clinical isolates to validate the performance of the method. Regular calibration of equipment and careful adherence to the CLSI or EUCAST guidelines are paramount.
The Role of the Kirby-Bauer Method in Combating Antibiotic Resistance
The Kirby-Bauer method plays a pivotal role in the fight against antibiotic resistance. By providing rapid and relatively inexpensive susceptibility testing, it guides clinicians in choosing appropriate antibiotics, thus minimizing the use of ineffective agents. This targeted approach reduces the selection pressure that drives the emergence and spread of antibiotic-resistant bacteria.
Future Directions and Advancements
While the Kirby-Bauer method remains a cornerstone of antibiotic susceptibility testing, ongoing research aims to improve its accuracy and efficiency. Automated systems are being developed to streamline the process and reduce human error. Moreover, research is focused on developing new techniques for detecting and characterizing antibiotic resistance mechanisms, which can complement the Kirby-Bauer method to enhance the understanding of resistance patterns.
Conclusion
The Kirby-Bauer method is a valuable and indispensable tool in microbiology laboratories worldwide. Its simplicity, cost-effectiveness, and ability to provide crucial information for guiding antibiotic therapy make it a cornerstone of infection control and antimicrobial stewardship programs. Understanding the principles, procedure, interpretation, and limitations of the Kirby-Bauer method is essential for anyone involved in microbiology, infectious disease management, and the global fight against antibiotic resistance. The continued refinement and adaptation of this method will undoubtedly play a significant role in shaping future strategies for combating antimicrobial resistance.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Rectangular Prism
Mar 20, 2025
-
What Are The Two Starting Materials For A Robinson Annulation
Mar 20, 2025
-
Living Things That Respond To Their Environment
Mar 20, 2025
-
Elements Of Group 17 Are Called
Mar 20, 2025
-
Is A Polymer Of Amino Acids
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Kirby Bauer Method Of Antibiotic Sensitivity Testing . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
