What Number Uniquely Identifies An Element
Muz Play
Mar 20, 2025 · 5 min read
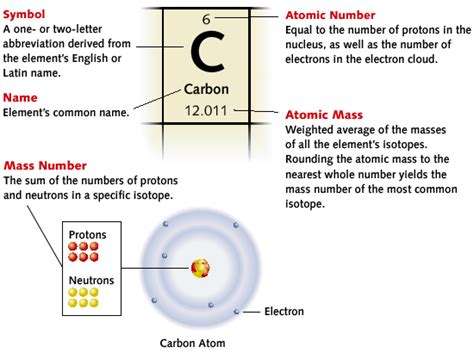
Table of Contents
What Number Uniquely Identifies an Element? The Atomic Number and Beyond
The question, "What number uniquely identifies an element?" has a straightforward answer: the atomic number. This fundamental concept in chemistry underpins our understanding of the periodic table and the behavior of matter. But the story doesn't end there. While the atomic number is the primary identifier, a deeper dive reveals nuances and related concepts that contribute to a complete understanding of elemental uniqueness. This article will explore the atomic number in detail, examine its significance, and touch upon other numerical identifiers that provide further insights into an element's characteristics.
The Atomic Number: The Defining Characteristic of an Element
The atomic number, represented by the symbol Z, represents the number of protons in the nucleus of an atom. This is the single most important characteristic that distinguishes one element from another. All atoms of a given element have the same atomic number; this is the defining feature. For example, all hydrogen atoms have an atomic number of 1 (one proton), all helium atoms have an atomic number of 2 (two protons), and so on.
Why is the atomic number so crucial?
- Defines the element: As stated earlier, it's the defining characteristic. A change in the atomic number fundamentally changes the element.
- Determines chemical properties: The number of protons dictates the number of electrons in a neutral atom. The arrangement of these electrons in electron shells determines the atom's chemical properties and how it interacts with other atoms.
- Uniquely identifies isotopes: While isotopes of an element have varying numbers of neutrons, they all share the same atomic number, distinguishing them as belonging to the same element.
Understanding Isotopes and Mass Number
While the atomic number remains constant for a given element, the number of neutrons in the nucleus can vary. Atoms of the same element with different numbers of neutrons are called isotopes. The mass number, often represented by A, is the total number of protons and neutrons in the nucleus.
For example, carbon has an atomic number of 6. The most common isotope is Carbon-12 (¹²C), which has 6 protons and 6 neutrons (A = 12). However, Carbon-14 (¹⁴C), a radioactive isotope used in carbon dating, has 6 protons and 8 neutrons (A = 14). Both are carbon because they both have 6 protons (Z=6), but they differ in their neutron count and thus their mass.
Beyond the Atomic Number: Other Numerical Identifiers
While the atomic number is paramount, other numerical properties provide additional information about an element and its behavior:
1. Mass Number (A):
As discussed, the mass number (A) is the sum of protons and neutrons. While it doesn't uniquely identify an element, it helps distinguish isotopes of the same element. It's crucial in nuclear physics and various applications like radioisotope dating and nuclear medicine. Knowing the mass number allows us to understand the stability and behavior of specific isotopes.
2. Atomic Mass/Weight:
Atomic mass is the average mass of all the isotopes of an element, weighted by their relative abundance. This is different from the mass number, which refers to a single isotope. The atomic mass is found on the periodic table and reflects the average mass of the element as it naturally occurs. This weighted average accounts for the fact that most elements exist as a mixture of isotopes.
3. Oxidation Number:
The oxidation number (also called oxidation state) indicates the apparent charge of an atom in a molecule or ion. It's not a fundamental property like the atomic number but reflects an atom's role in chemical bonding. It helps predict chemical reactions and understand the behavior of elements in different compounds.
4. Electron Configuration:
The arrangement of electrons in an atom's energy levels is described by its electron configuration. It’s not a single number but rather a notation that provides crucial insight into the chemical reactivity and bonding behavior of an element. This configuration is directly related to the atomic number as the number of electrons in a neutral atom is equal to the number of protons (atomic number).
5. CAS Registry Number:
The Chemical Abstracts Service (CAS) Registry Number is a unique numerical identifier assigned to every chemical substance, including elements, by the CAS, a division of the American Chemical Society. It's a crucial tool in chemical databases for unambiguous identification and information retrieval. While not a fundamental property of the element itself, it’s an extremely important numerical identifier in the context of chemical information management.
The Importance of Atomic Number in the Periodic Table
The periodic table's organization hinges on the atomic number. Elements are arranged in increasing order of atomic number, reflecting the systematic progression of chemical properties. The periodic table's structure, with its rows (periods) and columns (groups), reveals recurring trends in elemental properties, enabling us to predict the behavior of elements based on their position. This organization is directly linked to the electron configurations dictated by the atomic number, which in turn influence chemical behavior.
The periodic table is a powerful tool because it organizes elements based on the underlying principle of atomic number. Understanding this fundamental relationship is key to predicting reactivity, bond formation, and other important characteristics of elements.
Conclusion: Atomic Number as the Cornerstone of Elemental Identification
In conclusion, the atomic number (Z) is the definitive number that uniquely identifies an element. It dictates the element's fundamental properties, governs its chemical behavior, and underpins the organization of the periodic table. While other numerical identifiers, such as mass number, atomic mass, and CAS Registry Number, provide additional information about an element and its isotopes, none possess the fundamental significance of the atomic number in defining what an element is. Understanding the atomic number is essential for comprehending the structure and behavior of matter and unlocking the secrets of chemistry. The atomic number is not just a number; it’s the key to understanding the elements and their place in the universe. Further exploration of these related numerical identifiers enhances our understanding of the complexity and diversity within the world of elements.
Latest Posts
Latest Posts
-
What Is The Monomers Of Nucleic Acids
Mar 20, 2025
-
Bundles Of Axons Within The Central Nervous System Are Called
Mar 20, 2025
-
What Is The Basic Unit Of Heredity
Mar 20, 2025
-
Why Is Equatorial More Stable Than Axial
Mar 20, 2025
-
Chemistry A Molecular Approach Nivaldo Tro
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Number Uniquely Identifies An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
