What Is The Monomers Of Nucleic Acids
Muz Play
Mar 20, 2025 · 5 min read
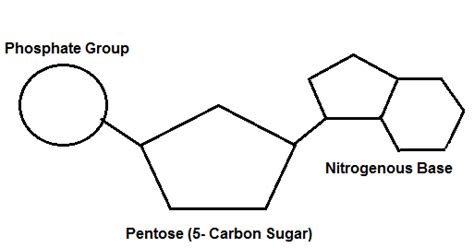
Table of Contents
What are the Monomers of Nucleic Acids? A Deep Dive into Nucleotides
Nucleic acids, the fundamental building blocks of life, are responsible for storing and transmitting genetic information. Understanding their structure is crucial to comprehending the intricacies of life itself. This article delves deep into the monomers of nucleic acids, exploring their composition, variations, and their critical role in biological processes.
The Building Blocks: Nucleotides – The Monomers of Nucleic Acids
The monomers of nucleic acids are nucleotides. These aren't just simple units; they are complex molecules composed of three essential components:
-
A Pentose Sugar: A five-carbon sugar molecule. This sugar is either ribose (in ribonucleic acid, or RNA) or deoxyribose (in deoxyribonucleic acid, or DNA). The difference lies in the presence or absence of a hydroxyl (-OH) group on the 2' carbon atom. This seemingly small difference has profound implications for the structure and function of the nucleic acid.
-
A Nitrogenous Base: This is a heterocyclic organic molecule containing nitrogen atoms. There are five main nitrogenous bases found in nucleic acids: adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U). Adenine and guanine are purines, characterized by a double-ring structure, while cytosine, thymine, and uracil are pyrimidines, possessing a single-ring structure. Thymine is found only in DNA, while uracil replaces thymine in RNA.
-
A Phosphate Group: This is a negatively charged group consisting of a phosphorus atom bonded to four oxygen atoms. The phosphate group plays a crucial role in linking nucleotides together to form the polynucleotide chains of DNA and RNA. It also contributes to the overall negative charge of nucleic acids.
Detailed Examination of Each Component
Let's delve deeper into each of these components:
1. Pentose Sugars:
-
Ribose (in RNA): The presence of the 2'-hydroxyl group in ribose makes RNA more reactive and less stable than DNA. This increased reactivity is partly responsible for RNA's roles in catalysis and transient information transfer. The extra hydroxyl group makes RNA more susceptible to hydrolysis, a process where water breaks the bonds, leading to degradation.
-
Deoxyribose (in DNA): The absence of the 2'-hydroxyl group in deoxyribose makes DNA more stable and less prone to hydrolysis. This stability is crucial for the long-term storage of genetic information, which DNA is primarily responsible for. The increased stability contributes to the integrity of the genome over time.
2. Nitrogenous Bases:
The nitrogenous bases are responsible for the specificity of genetic information. The sequence of these bases along the nucleic acid chain determines the genetic code.
-
Purines (Adenine and Guanine): These double-ringed structures are larger than pyrimidines. Their specific hydrogen bonding patterns are crucial for base pairing in DNA and RNA.
-
Pyrimidines (Cytosine, Thymine, and Uracil): These single-ringed structures are smaller than purines. The different pyrimidines, specifically the presence of thymine in DNA versus uracil in RNA, contributes to the structural differences and functional distinctions between the two nucleic acids.
3. Phosphate Group:
The phosphate group forms the backbone of the nucleic acid polymer. It links the 3' carbon of one sugar to the 5' carbon of the next sugar through phosphodiester bonds. This creates a directional chain with a 5' end (with a free phosphate group) and a 3' end (with a free hydroxyl group). The negative charge of the phosphate group also contributes to the solubility of nucleic acids in water.
Nucleotide Formation and Nomenclature
Nucleotides are formed through a series of enzymatic reactions. First, the nitrogenous base is attached to the pentose sugar, forming a nucleoside. Then, a phosphate group is added to the nucleoside, creating a nucleotide.
The nomenclature of nucleotides is systematic. For example:
- Adenosine monophosphate (AMP): Adenine + ribose + one phosphate group
- Guanosine triphosphate (GTP): Guanine + ribose + three phosphate groups
- Deoxythymidine monophosphate (dTMP): Thymine + deoxyribose + one phosphate group
The presence of one, two, or three phosphate groups is indicated by the prefixes mono-, di-, or tri-, respectively. The "d" prefix before the nucleotide name denotes that it contains deoxyribose instead of ribose.
The Significance of Nucleotide Variation
The variations in the components of nucleotides – the sugar, the base, and the number of phosphate groups – are not trivial. They lead to diverse functions in the cell:
-
Energy Currency: Nucleotide triphosphates, such as ATP (adenosine triphosphate) and GTP, are the primary energy currency of the cell. The hydrolysis of the high-energy phosphate bonds releases energy that fuels numerous cellular processes.
-
Signal Transduction: Cyclic nucleotides, such as cyclic AMP (cAMP) and cyclic GMP (cGMP), act as second messengers in signal transduction pathways, relaying information from cell surface receptors to intracellular targets.
-
Coenzymes: Some nucleotides serve as coenzymes, assisting enzymes in catalyzing biochemical reactions. For example, NAD+ (nicotinamide adenine dinucleotide) and FAD (flavin adenine dinucleotide) are essential coenzymes in cellular respiration.
-
Building Blocks of Nucleic Acids: Ultimately, nucleotides are the essential monomers that make up DNA and RNA, enabling the storage and transmission of genetic information, the basis for life as we know it.
The Polymerization of Nucleotides: Forming Nucleic Acid Chains
Nucleotides are linked together through phosphodiester bonds to form the polynucleotide chains of DNA and RNA. The 3' hydroxyl group of one nucleotide reacts with the 5' phosphate group of the next nucleotide, releasing a water molecule. This process is catalyzed by enzymes called DNA polymerases (for DNA) and RNA polymerases (for RNA). The resulting chain has a distinct 5' to 3' polarity.
The sequence of nucleotides in a nucleic acid chain dictates the genetic information. The specific order of the four bases (A, T, C, G in DNA; A, U, C, G in RNA) forms the genetic code that determines the amino acid sequence of proteins and controls various cellular functions.
Conclusion: Nucleotides - The Foundation of Life
In conclusion, nucleotides, the monomers of nucleic acids, are not simply building blocks but highly versatile molecules essential for life. Their composition – a pentose sugar, a nitrogenous base, and a phosphate group – dictates their properties and functions. Understanding the diverse roles of nucleotides, from energy transfer to genetic information storage, is critical for comprehending the fundamental processes of life. The variations in the sugar, base, and phosphate groups lead to a range of functions within the cell, highlighting the elegant simplicity and complexity of this fundamental biological molecule. The sequence of nucleotides in DNA and RNA forms the basis of the genetic code, the instructions for life itself. Further research into the intricacies of nucleotide structure and function continues to unveil new insights into the complexities of biological systems.
Latest Posts
Latest Posts
-
Law Of Segregation Vs Law Of Independent Assortment
Mar 20, 2025
-
Are Substances With A High Melting Point Soluble
Mar 20, 2025
-
Members Of The Kingdom Fungi Are Photosynthetic
Mar 20, 2025
-
Organic Chemistry Substitution And Elimination Reactions Practice Problems
Mar 20, 2025
-
What Is Gas To Solid Called
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Monomers Of Nucleic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
