Do Acids Gain Or Lose Hydrogen Ions
Muz Play
Mar 19, 2025 · 6 min read
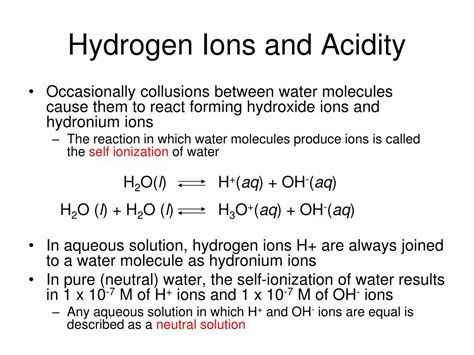
Table of Contents
Do Acids Gain or Lose Hydrogen Ions? Understanding Acid-Base Chemistry
The question of whether acids gain or lose hydrogen ions is fundamental to understanding acid-base chemistry. The answer, simply put, is that acids lose hydrogen ions (H⁺). This seemingly straightforward concept underpins a vast array of chemical reactions and biological processes. Let's delve deeper into the intricacies of this process, exploring the different definitions of acids, the mechanisms of hydrogen ion donation, and the consequences of this ion transfer.
Defining Acids: Arrhenius, Brønsted-Lowry, and Lewis Theories
Before examining the behavior of hydrogen ions in acids, it's crucial to understand the various definitions of acids themselves. Three prominent theories provide different perspectives:
1. Arrhenius Theory
The Arrhenius theory, one of the earliest definitions, defines an acid as a substance that increases the concentration of hydrogen ions (H⁺) in an aqueous solution. This theory is simple and effective for many common acids, such as hydrochloric acid (HCl) which dissociates in water to form H⁺ and Cl⁻ ions. However, it has limitations, as it only applies to aqueous solutions and doesn't encompass all substances exhibiting acidic properties.
2. Brønsted-Lowry Theory
The Brønsted-Lowry theory offers a broader definition. It defines an acid as a proton (H⁺) donor. This definition extends beyond aqueous solutions, encompassing reactions in non-aqueous solvents or even gas-phase reactions. Crucially, this theory introduces the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. For example, when HCl donates a proton, it becomes its conjugate base, Cl⁻. This theory is more comprehensive than the Arrhenius theory and better explains the behavior of acids in a wider range of contexts.
3. Lewis Theory
The Lewis theory provides the most general definition of an acid. A Lewis acid is defined as an electron pair acceptor. This definition encompasses substances that don't necessarily contain hydrogen but can still accept an electron pair, thereby exhibiting acidic characteristics. Examples include boron trifluoride (BF₃) and aluminum chloride (AlCl₃). While not directly related to hydrogen ion donation, Lewis acids often participate in reactions that involve the redistribution of electrons, which can indirectly influence the behavior of hydrogen ions in other parts of the reaction system.
The Mechanism of Hydrogen Ion Donation
The process of hydrogen ion donation, central to the Brønsted-Lowry definition, is driven by the relative stability of the species involved. Strong acids readily donate their proton, while weak acids do so less readily.
Strong Acids vs. Weak Acids
Strong acids completely dissociate in water, meaning they lose their hydrogen ion almost entirely. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃). The resulting ions are highly stable, making the proton donation essentially irreversible in aqueous solutions.
Weak acids, on the other hand, only partially dissociate in water. This means that only a small fraction of the acid molecules donate their proton. The equilibrium lies far to the left, indicating that a significant portion of the acid remains undissociated. Examples include acetic acid (CH₃COOH) and carbonic acid (H₂CO₃). The resulting ions are less stable, and the proton donation is reversible. This equilibrium is described by an acid dissociation constant (Ka), a measure of the acid's strength. A higher Ka value indicates a stronger acid.
Factors Affecting Hydrogen Ion Donation
Several factors influence the ease with which an acid donates a hydrogen ion:
- Electronegativity: The electronegativity of the atom bonded to the hydrogen atom plays a significant role. A more electronegative atom pulls electron density away from the hydrogen, making it easier to release as a proton.
- Bond Strength: A weaker bond between the hydrogen and the rest of the molecule facilitates proton donation.
- Solvent Effects: The solvent in which the acid is dissolved significantly impacts its dissociation. Polar solvents, like water, stabilize the resulting ions, promoting dissociation.
- Resonance Stabilization: If the conjugate base formed after proton donation can be stabilized through resonance, the acid will be stronger, more readily donating its proton.
Consequences of Hydrogen Ion Loss
The loss of hydrogen ions by acids has profound consequences:
- Lowering pH: The increase in H⁺ ion concentration in a solution directly lowers its pH, making it more acidic. The pH scale is logarithmic, meaning a change of one pH unit represents a tenfold change in H⁺ ion concentration.
- Chemical Reactions: The released hydrogen ions can participate in various chemical reactions, acting as reactants or catalysts. For instance, in neutralization reactions, hydrogen ions react with hydroxide ions (OH⁻) to form water.
- Biological Processes: Hydrogen ion concentration plays a critical role in many biological processes. Enzymes, for example, often have optimal pH ranges within which they function efficiently. Changes in H⁺ concentration can significantly impact enzyme activity and overall metabolic processes.
Examples of Acids Losing Hydrogen Ions
Let's examine some specific examples:
1. Hydrochloric Acid (HCl): A strong acid, HCl completely dissociates in water:
HCl(aq) → H⁺(aq) + Cl⁻(aq)
The hydrogen ion is readily donated, leading to a highly acidic solution.
2. Acetic Acid (CH₃COOH): A weak acid, acetic acid only partially dissociates:
CH₃COOH(aq) ⇌ CH₃COO⁻(aq) + H⁺(aq)
The equilibrium lies to the left, meaning most of the acetic acid remains undissociated.
3. Citric Acid (C₆H₈O₇): A triprotic weak acid, citric acid can donate three protons in a stepwise manner, each with a different Ka value reflecting the varying ease of proton donation at each step.
4. Carbonic Acid (H₂CO₃): A diprotic weak acid, important in blood pH regulation, carbonic acid donates protons in two steps. The equilibrium of each step plays a vital role in maintaining the proper pH balance in the blood.
Conclusion: Acids as Proton Donors
In summary, acids, according to the Brønsted-Lowry definition, are proton (H⁺) donors. They lose hydrogen ions, leading to a decrease in pH and influencing a wide range of chemical and biological processes. The strength of an acid determines the extent to which it loses its hydrogen ions, with strong acids completely dissociating and weak acids only partially dissociating. Understanding this fundamental principle is crucial for comprehending a vast array of chemical and biological phenomena. The intricacies of acid-base chemistry, including the various factors influencing proton donation and the consequences of hydrogen ion release, are continually researched and refined, constantly expanding our understanding of this fundamental aspect of chemistry. The three theories—Arrhenius, Brønsted-Lowry, and Lewis—provide a progressively more comprehensive understanding of the behavior of acids, showcasing the evolution of scientific thought in this field. The interplay between these theories highlights the dynamic and ever-evolving nature of scientific understanding.
Latest Posts
Latest Posts
-
What Elements Are Most Likley To Become Cations
Mar 19, 2025
-
What Is A Medium In Writing
Mar 19, 2025
-
The Starting Substances In A Chemical Reaction
Mar 19, 2025
-
Energy Diagram For A Two Step Reaction
Mar 19, 2025
-
Right Hand Rule For Angular Momentum
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Gain Or Lose Hydrogen Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
