The Starting Substances In A Chemical Reaction
Muz Play
Mar 19, 2025 · 6 min read
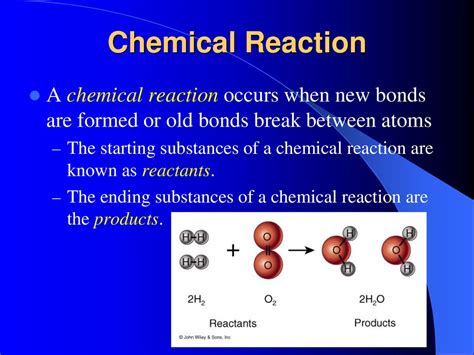
Table of Contents
The Starting Substances in a Chemical Reaction: Reactants and Their Roles
Understanding the starting substances in a chemical reaction is fundamental to grasping the entire process. These substances, known as reactants, are the key players that undergo transformation to produce new substances called products. This article delves deep into the nature of reactants, exploring their diverse roles, characteristics, and influence on the reaction's outcome. We'll examine factors like stoichiometry, limiting reactants, and the impact of reactant properties on reaction kinetics and equilibrium.
What are Reactants?
Reactants are the chemical species that are consumed during a chemical reaction. They are the initial ingredients that enter into the reaction process, undergoing changes in their chemical bonds and structure to form the products. Think of baking a cake: the flour, sugar, eggs, and butter are the reactants, combining to create the cake (the product). In a chemical equation, reactants are written on the left-hand side of the arrow.
Example: The combustion of methane (CH₄) with oxygen (O₂) to produce carbon dioxide (CO₂) and water (H₂O) can be represented as:
CH₄ + 2O₂ → CO₂ + 2H₂O
Here, methane (CH₄) and oxygen (O₂) are the reactants.
Types of Reactants
Reactants are not monolithic; they exist in diverse forms and play various roles within a reaction. We can broadly categorize them based on several factors:
-
By their chemical nature: Reactants can be elements, compounds, or ions. For example, in the rusting of iron (Fe), the reactant is elemental iron reacting with oxygen (O₂) and water (H₂O).
-
By their function: Sometimes, reactants act as catalysts or inhibitors, affecting the reaction rate without being consumed themselves. Catalysts speed up reactions, while inhibitors slow them down. Enzymes, biological catalysts, are prime examples of reactants that function as catalysts.
-
By their state of matter: Reactants can be solids, liquids, gases, or even exist in solution. The state of matter significantly impacts the reaction rate and mechanism. For instance, a reaction between a solid and a liquid will proceed differently than a reaction between two gases.
Stoichiometry and Reactant Ratios
Stoichiometry is the quantitative relationship between reactants and products in a chemical reaction. It describes the precise ratio in which reactants combine and products are formed. This ratio is determined by the balanced chemical equation.
In the methane combustion example above, the stoichiometric ratio of methane to oxygen is 1:2. This means that for every one molecule of methane, two molecules of oxygen are required for complete combustion. Any deviation from this ratio can lead to incomplete combustion and the formation of unwanted byproducts.
Limiting Reactants and Excess Reactants
In many reactions, the reactants are not present in stoichiometric proportions. One reactant will be completely consumed before the others, limiting the amount of product that can be formed. This reactant is called the limiting reactant. The other reactants present in excess are called excess reactants. Identifying the limiting reactant is crucial for calculating the theoretical yield of a reaction.
Example: Consider a reaction where 2 moles of A react with 1 mole of B to produce C:
2A + B → C
If we have 3 moles of A and 1 mole of B, B is the limiting reactant because it will be fully consumed before A. Only 0.5 moles of C will be formed. 1.5 moles of A will remain unreacted.
Factors Influencing Reactant Behavior
Several factors significantly impact the behavior of reactants and the overall reaction:
-
Concentration: Higher reactant concentrations generally lead to faster reaction rates, as there are more reactant particles available to collide and react.
-
Temperature: Increasing temperature usually increases the reaction rate. Higher temperatures provide more kinetic energy to the reactant particles, increasing the frequency and energy of collisions, thus leading to a higher likelihood of reaction.
-
Pressure (for gases): Increased pressure for gaseous reactants increases their concentration, leading to faster reaction rates (similar to concentration effects).
-
Surface area (for solids): For reactions involving solids, a larger surface area exposes more reactant particles to react, enhancing the reaction rate. Powdered reactants react faster than solid chunks.
-
Catalyst presence: Catalysts accelerate reactions by providing an alternative reaction pathway with a lower activation energy. They do not get consumed during the reaction and can be recovered at the end.
-
Solvent effects: The solvent used in a reaction can significantly influence the reaction rate and equilibrium by altering the solubility and interactions of the reactants.
Reactants and Reaction Mechanisms
Understanding the detailed steps by which a reaction proceeds is crucial. This is encapsulated by the reaction mechanism. Reactants play a central role in shaping the mechanism. The sequence of elementary steps, including bond breaking and formation, is dependent on the nature of the reactant molecules and their interactions.
For example, some reactions proceed through a series of intermediate steps involving reactive intermediates, which are short-lived species formed during the reaction process. The structure and reactivity of these intermediates are directly influenced by the starting reactants.
Reactants and Reaction Equilibrium
Chemical reactions are often reversible; they can proceed in both the forward and reverse directions. When the rates of the forward and reverse reactions become equal, the system reaches equilibrium. The relative concentrations of reactants and products at equilibrium are described by the equilibrium constant (K). The value of K indicates the extent to which the reaction proceeds to completion.
The initial concentrations of the reactants significantly impact the position of equilibrium. If the initial concentration of reactants is high, the reaction will tend to shift towards the formation of products to achieve equilibrium.
Characterizing Reactants
The properties of reactants significantly affect the overall reaction. Key aspects include:
-
Chemical structure: The arrangement of atoms and bonds within the reactant molecule profoundly influences its reactivity. Functional groups, stereochemistry, and resonance effects are crucial considerations.
-
Reactivity: Some reactants are highly reactive, while others are less so. This inherent reactivity stems from the electronic structure and bonding within the molecule.
-
Solubility: The solubility of reactants in a particular solvent can dictate the reaction rate and efficiency. Reactants need to be adequately soluble to effectively interact.
-
Stability: The stability of reactants reflects their tendency to undergo transformation. Less stable reactants tend to react more readily.
-
Purity: Impurities in reactants can significantly impact the reaction outcome, sometimes leading to unwanted side products or inhibiting the main reaction.
Analyzing Reactants: Experimental Techniques
Various experimental techniques are employed to analyze reactants and study their behavior. These include:
-
Spectroscopy (NMR, IR, UV-Vis): These techniques provide detailed information about the structure, functional groups, and electronic properties of the reactants.
-
Chromatography (GC, HPLC): These separation techniques are used to determine the purity of reactants and identify any impurities present.
-
Titration: Titration is a quantitative technique to determine the concentration of a reactant.
-
Mass Spectrometry: This technique allows for the precise determination of the molecular weight and composition of reactants.
Conclusion
The starting substances, the reactants, are the cornerstone of any chemical reaction. Their properties, stoichiometric ratios, and interactions define the reaction's progress, rate, and equilibrium. Understanding these aspects is essential not just for predicting reaction outcomes but also for designing and optimizing chemical processes across various disciplines, from industrial chemistry to biochemistry and beyond. The detailed analysis of reactants, using a combination of theoretical understanding and experimental techniques, remains a crucial endeavor in the field of chemistry.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Sodium Chloride
Mar 20, 2025
-
What Number Uniquely Identifies An Element
Mar 20, 2025
-
An Atom That Has Gained Or Lost Electrons
Mar 20, 2025
-
Compounds Containing Only Carbon And Hydrogen Are Called
Mar 20, 2025
-
How Many Covalent Bonds Does Hydrogen Have
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about The Starting Substances In A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
