What Elements Are Most Likley To Become Cations
Muz Play
Mar 19, 2025 · 6 min read
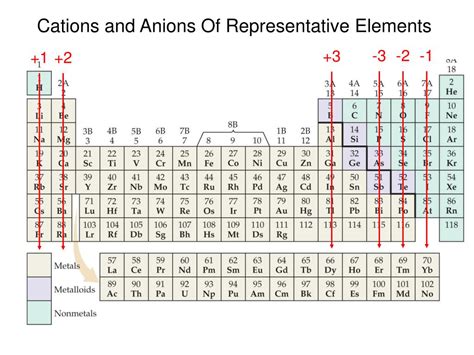
Table of Contents
What Elements Are Most Likely to Become Cations?
Understanding which elements readily form cations—positively charged ions—is fundamental to chemistry. This knowledge is crucial for predicting chemical reactions, understanding bonding, and interpreting the properties of various compounds. This comprehensive article delves into the factors that determine an element's propensity to lose electrons and become a cation, exploring periodic trends, electronegativity, ionization energy, and the role of electron configuration.
Understanding Cations and Ionization
A cation is formed when a neutral atom loses one or more electrons, resulting in a net positive charge. This loss of electrons is driven by the element's inherent properties and its interaction with other atoms or molecules. The process of removing an electron from a neutral atom is called ionization, and the energy required to do so is called the ionization energy.
Factors Influencing Cation Formation
Several key factors influence an element's likelihood of forming cations:
-
Low Ionization Energy: Elements with low ionization energies readily lose electrons because less energy is needed to overcome the electrostatic attraction between the nucleus and the valence electrons. This means the outer electrons are relatively loosely held.
-
Electron Configuration: Elements with valence electron configurations close to a stable noble gas configuration (eight electrons in the outermost shell, except for helium with two) are more likely to lose electrons to achieve this stable state. This follows the octet rule, a guiding principle in chemical bonding.
-
Electronegativity: Electronegativity measures an atom's ability to attract electrons towards itself in a chemical bond. Elements with low electronegativity are less likely to attract electrons and are more likely to lose them, forming cations. This is inversely related to the tendency to form cations; lower electronegativity implies a higher likelihood of cation formation.
-
Metallic Character: Metallic character is the tendency of an element to exhibit properties characteristic of metals, such as conductivity and malleability. Metals generally have low ionization energies and readily lose electrons to form cations. The metallic character increases as you move down and to the left of the periodic table.
Periodic Trends and Cation Formation
The periodic table organizes elements based on their atomic structure and properties, revealing clear trends in cation formation:
Group 1 (Alkali Metals): The Most Ready Cation Formers
Alkali metals (Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr)) are the most electropositive elements. They have only one electron in their outermost shell, making it exceptionally easy to lose this electron and achieve a stable noble gas configuration. They consistently form +1 cations (e.g., Na⁺, K⁺). Their low ionization energies further contribute to their strong tendency to form cations.
Group 2 (Alkaline Earth Metals): Relatively Easy Cation Formation
Alkaline earth metals (Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra)) have two electrons in their outermost shell. They readily lose these two electrons to achieve a stable configuration, forming +2 cations (e.g., Mg²⁺, Ca²⁺). While their ionization energies are higher than alkali metals, they still readily form cations, particularly in reactions with highly electronegative elements.
Transition Metals: Variable Oxidation States
Transition metals occupy the d-block of the periodic table. They exhibit variable oxidation states, meaning they can lose different numbers of electrons to form cations with different charges. This variability arises from the relatively close energy levels of their d and s electrons, allowing them to lose electrons from either shell depending on the reaction conditions and the electronegativity of the interacting element. For example, iron (Fe) can form Fe²⁺ and Fe³⁺ cations. The specific cation formed often depends on the oxidizing agent and the reaction environment.
Post-Transition Metals: A Mixed Bag
Post-transition metals (e.g., Aluminum (Al), Tin (Sn), Lead (Pb)) are located to the right of the transition metals. Their behavior is less predictable than alkali and alkaline earth metals. While they tend to form cations, they can also exhibit some covalent character in their bonding. For instance, aluminum usually forms a +3 cation (Al³⁺), but its compounds can display some degree of covalent bonding characteristics.
Poor Metals and Metalloids: Less Likely to Form Cations
Moving further to the right of the periodic table, elements exhibit increasingly non-metallic behavior. Poor metals and metalloids (e.g., arsenic, antimony, tellurium) have higher ionization energies and are less likely to form simple cations. They frequently form covalent compounds or complex ions involving more intricate bonding arrangements.
Nonmetals: Rare Cation Formation
Nonmetals (e.g., halogens, oxygen, nitrogen) are strongly electronegative and generally gain electrons to achieve a stable octet, forming anions (negatively charged ions) rather than cations. However, under specific and often extreme conditions, some nonmetals can exist in cationic forms. For instance, nitrogen can form the N⁺ ion in specific compounds.
Implications of Cation Formation
The formation of cations has significant implications across various areas of chemistry:
-
Ionic Compounds: Cations are essential building blocks of ionic compounds, where they are electrostatically attracted to anions to form a crystal lattice. The properties of ionic compounds (e.g., melting point, solubility) are largely dictated by the charge and size of the constituent cations and anions.
-
Chemical Reactions: The tendency of an element to form cations dictates its reactivity. Elements that easily form cations are often highly reactive, readily participating in redox reactions (reactions involving electron transfer).
-
Coordination Chemistry: Transition metal cations are central to coordination chemistry, where they form complexes with ligands (molecules or ions that donate electrons). The properties and reactivity of these complexes depend on the cation's charge, size, and electron configuration.
-
Biological Systems: Many biological processes involve the transport and interaction of cations, such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions. These ions play crucial roles in nerve impulse transmission, muscle contraction, and enzyme activity.
Beyond Simple Cation Formation: Polyatomic Ions
It's crucial to note that cation formation isn't limited to single atoms. Polyatomic ions are charged groups of atoms that act as a single unit. These ions can be positively charged (polyatomic cations) or negatively charged (polyatomic anions). Examples of polyatomic cations include the ammonium ion (NH₄⁺) and hydronium ion (H₃O⁺). The formation of polyatomic ions involves covalent bonding within the ion, but the ion as a whole carries a net positive charge and behaves similarly to simple cations in many chemical contexts.
Conclusion
The likelihood of an element forming a cation is primarily determined by its ionization energy, electron configuration, electronegativity, and position within the periodic table. Alkali and alkaline earth metals are the most likely to form cations, while nonmetals generally avoid cation formation. Understanding these factors is crucial for predicting chemical behavior, interpreting reaction mechanisms, and comprehending the properties of various compounds and materials. The concept of cation formation extends beyond single atoms, encompassing polyatomic ions that play vital roles in chemistry and biology. Further exploration of these principles provides a deeper understanding of the fundamental forces shaping the chemical world.
Latest Posts
Latest Posts
-
Internal Anatomy Of A Bony Fish
Mar 20, 2025
-
What Is The Major Drawback To Using Word Equations
Mar 20, 2025
-
What Are The Reactants In Fermentation
Mar 20, 2025
-
Why Is Ice Less Dense Than Liquid Water
Mar 20, 2025
-
Drawing Of Law Of Conservation Of Matter
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Elements Are Most Likley To Become Cations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
