Why Is Ice Less Dense Than Liquid Water
Muz Play
Mar 20, 2025 · 6 min read
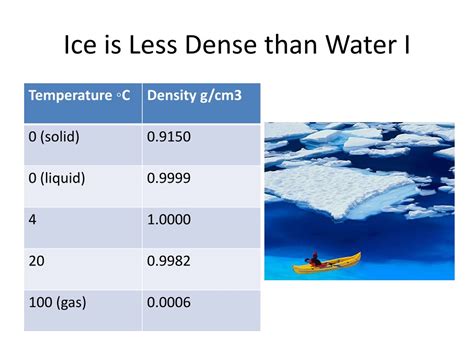
Table of Contents
Why is Ice Less Dense Than Liquid Water? An In-Depth Explanation
Water is unique. It's a substance we interact with constantly, yet its properties often defy our expectations. One of the most remarkable characteristics of water is that its solid form, ice, is less dense than its liquid form. This seemingly simple fact has profound consequences for life on Earth, shaping our planet's climate, ecosystems, and even the very possibility of life as we know it. Let's delve deep into the fascinating science behind this unusual property.
The Role of Hydrogen Bonds
The answer lies in the structure of water molecules and the powerful forces that bind them together: hydrogen bonds. A water molecule (H₂O) consists of two hydrogen atoms covalently bonded to a single oxygen atom. Oxygen is more electronegative than hydrogen, meaning it attracts the shared electrons more strongly. This creates a slight negative charge (δ-) on the oxygen atom and slight positive charges (δ+) on the hydrogen atoms, making the water molecule polar.
This polarity is crucial because it allows water molecules to form hydrogen bonds with each other. The slightly positive hydrogen atom of one water molecule is attracted to the slightly negative oxygen atom of a neighboring molecule. These hydrogen bonds are relatively weak compared to covalent bonds, but they are numerous and collectively exert a significant influence on the properties of water.
The Crystalline Structure of Ice
In liquid water, the hydrogen bonds are constantly breaking and reforming as molecules move around freely. This creates a relatively disordered structure. However, as water freezes, the molecules become more ordered, aligning themselves into a specific crystalline structure.
This structure is characterized by a tetrahedral arrangement, where each water molecule is surrounded by four other water molecules linked by hydrogen bonds. To maximize the number of hydrogen bonds, the molecules arrange themselves in a hexagonal lattice with relatively large spaces between them. This open structure is the key to ice's lower density.
Visualizing the Open Structure
Imagine building a structure with LEGO bricks. In liquid water, the bricks are jumbled together, with some spaces but a generally packed arrangement. In ice, however, the bricks are arranged in a specific, more spacious pattern. This pattern, while maximizing hydrogen bonds, leaves significant gaps, resulting in a less dense structure compared to the more randomly packed liquid state.
Density: Mass per Unit Volume
Density is defined as mass per unit volume (ρ = m/V). Since ice has the same mass as the equivalent volume of liquid water, its lower density implies it occupies a larger volume. The open crystalline structure of ice allows for more empty space between molecules, thus increasing the overall volume while maintaining the same mass. This is why ice floats on water.
Consequences of Ice's Lower Density
The fact that ice is less dense than water has several crucial consequences:
1. Insulation of Aquatic Life
In winter, ice forms on the surface of lakes and rivers. Because ice floats, it forms an insulating layer on top, preventing the underlying water from freezing solid. This allows aquatic life to survive in sub-zero temperatures. If ice were denser than water, it would sink to the bottom, leading to the complete freezing of water bodies and potentially devastating consequences for aquatic ecosystems.
2. Global Climate Regulation
The floating ice plays a critical role in regulating the Earth's climate. The reflective surface of ice and snow (albedo effect) reflects sunlight back into space, helping to maintain a relatively stable global temperature. Changes in the extent of ice cover, due to climate change, can significantly impact this effect and influence global temperatures.
3. Water's Unique Properties as a Solvent
The hydrogen bonding in water contributes to its exceptional ability to dissolve many substances, making it an excellent solvent. This property is essential for biological processes, as water acts as a medium for transporting nutrients and carrying out chemical reactions within living organisms. The open structure of ice, although seemingly unrelated, plays a part in these properties as the very nature of the hydrogen bonds themselves stem from this basic molecular arrangement.
4. Influence on Weather Patterns
The latent heat of fusion (the energy required to melt ice) and the latent heat of vaporization (the energy required to convert liquid water into vapor) are both relatively high compared to other substances. This means that water plays a significant role in moderating temperature fluctuations, influencing weather patterns and climate. The formation and melting of ice significantly impact these energy exchanges.
Comparing Ice and Liquid Water at the Molecular Level
The difference in density isn't merely a matter of macroscopic arrangement. Even at the molecular level, the dynamics are significantly different. In liquid water, the hydrogen bonds are constantly breaking and reforming, leading to a dynamic and constantly shifting network. This allows for a more compact arrangement despite individual bonds being constantly broken and remade.
In ice, however, the hydrogen bonds are more stable and fixed within the crystalline structure. While the stronger bond in ice creates a stable structure, this also means less freedom for individual molecules. This causes the molecules to adopt a more "spaced out" arrangement to maximize hydrogen bonds while maintaining stability, hence the lower density.
Factors Affecting Ice Density
While the basic tetrahedral structure is the primary reason for ice's lower density, several factors can slightly influence its density:
- Pressure: Increasing pressure forces water molecules closer together, compressing the ice structure and increasing its density. At extremely high pressures, different ice phases with higher densities can form.
- Temperature: The density of ice, like any solid, varies slightly with temperature. Lower temperatures generally lead to slightly higher density. It is important to note, however, that this is a minor effect compared to the significant density difference between liquid water and ice at standard conditions.
- Impurities: The presence of dissolved substances or impurities in the water can slightly affect the ice's crystalline structure and density, which can manifest in varying degrees of ice formation.
Conclusion: A Unique Phenomenon with Profound Implications
The fact that ice is less dense than liquid water is a remarkable exception in the natural world, a consequence of the unique properties of water molecules and the strong hydrogen bonds they form. This seemingly simple phenomenon has profound consequences for the planet's climate, ecosystems, and the existence of life itself. Understanding this anomaly is crucial to appreciating the complex and vital role water plays in shaping our world. From the survival of aquatic organisms to the regulation of global temperatures, the lower density of ice is a testament to the astonishing complexity and elegant design of our natural world. The study of water's unique properties continues to inspire scientific curiosity and provides valuable insights into the fundamentals of chemistry, physics, and biology. Further research into this area will undoubtedly reveal even deeper levels of understanding.
Latest Posts
Latest Posts
-
Bundles Of Axons Within The Central Nervous System Are Called
Mar 20, 2025
-
What Is The Basic Unit Of Heredity
Mar 20, 2025
-
Why Is Equatorial More Stable Than Axial
Mar 20, 2025
-
Chemistry A Molecular Approach Nivaldo Tro
Mar 20, 2025
-
Humidity Is Measured With What Instrument
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Why Is Ice Less Dense Than Liquid Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
