Is Alcohol A Acid And A Base Bronsted
Muz Play
Mar 19, 2025 · 5 min read
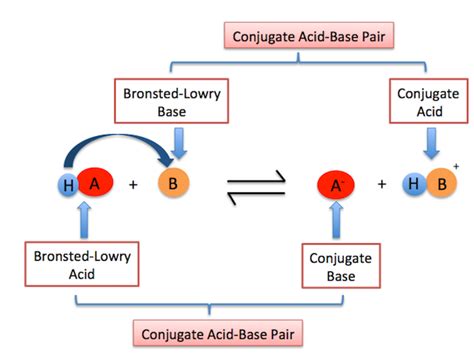
Table of Contents
Is Alcohol a Brønsted Acid and Base? A Deep Dive into Acid-Base Chemistry
The question of whether alcohol acts as a Brønsted acid or base is a nuanced one, requiring a thorough understanding of acid-base chemistry and the specific properties of alcohols. While not as straightforward as strong acids like HCl or strong bases like NaOH, alcohols exhibit amphoteric behavior under certain conditions, meaning they can act as both acids and bases, albeit weakly. This article will delve deep into the intricacies of alcohol's acid-base properties within the context of the Brønsted-Lowry theory.
Understanding the Brønsted-Lowry Definition
Before exploring the behavior of alcohols, let's solidify our understanding of the Brønsted-Lowry definition of acids and bases. According to this theory, a Brønsted-Lowry acid is a substance that donates a proton (H⁺ ion), while a Brønsted-Lowry base is a substance that accepts a proton. This definition expands upon the earlier Arrhenius definition by including substances that don't necessarily involve hydroxide (OH⁻) ions as bases. The key here is the proton transfer.
Acidic Behavior of Alcohols
Alcohols, with their general formula R-OH (where R is an alkyl group), possess a hydroxyl (-OH) group. The oxygen atom in this group is more electronegative than the hydrogen atom, creating a polar covalent bond. This polarity allows the hydrogen to be relatively easily removed as a proton. However, it's crucial to understand that alcohols are weak acids. They only partially dissociate in solution, meaning only a small fraction of the alcohol molecules donate a proton.
The acidity of alcohols is primarily determined by the stability of the alkoxide ion (RO⁻) formed after proton donation. The more stable the alkoxide ion, the more acidic the alcohol. Factors influencing alkoxide ion stability include:
-
Inductive Effect: Electron-withdrawing groups near the hydroxyl group can stabilize the negative charge on the alkoxide ion, increasing the acidity of the alcohol. For example, trifluoroethanol (CF₃CH₂OH) is significantly more acidic than ethanol (CH₃CH₂OH) due to the strong electron-withdrawing effect of the trifluoromethyl group.
-
Resonance: If the alkoxide ion can participate in resonance, the negative charge can be delocalized, further stabilizing the ion and increasing acidity. Phenols (aromatic alcohols) are a prime example; the phenoxide ion (formed from phenol) is resonance-stabilized, making phenols considerably more acidic than aliphatic alcohols.
-
Steric Effects: Bulky groups near the hydroxyl group can hinder the approach of a proton acceptor, decreasing the acidity.
Examples of Alcohol's Acidic Behavior:
Alcohols can react with strong bases like sodium hydride (NaH) or sodium amide (NaNH₂) to form alkoxide salts and hydrogen gas. This reaction demonstrates the acidic nature of the alcohol, even though it's a weak acid. The strong base deprotonates the alcohol, forming the conjugate base (alkoxide ion).
R-OH + NaH → R-O⁻Na⁺ + H₂
Basic Behavior of Alcohols
The lone pairs of electrons on the oxygen atom in the hydroxyl group of alcohols allow them to act as Brønsted-Lowry bases. They can accept a proton from a strong acid, forming an oxonium ion (R-OH₂⁺). However, like their acidic behavior, alcohols are weak bases. Their basicity is generally much weaker than their acidity.
Examples of Alcohol's Basic Behavior:
Alcohols can react with strong acids like sulfuric acid (H₂SO₄) or hydroiodic acid (HI). The oxygen atom in the alcohol accepts a proton from the acid, forming a protonated alcohol (oxonium ion). This protonated alcohol can then undergo further reactions, such as dehydration to form alkenes or substitution reactions.
R-OH + H⁺ → R-OH₂⁺
Factors Affecting Acidity and Basicity
Several factors influence the relative acidity and basicity of alcohols:
-
Alkyl Group Size: As the size of the alkyl group (R) increases, the acidity of the alcohol slightly decreases. This is due to the inductive effect of the alkyl group, which donates electron density to the oxygen atom, making it less likely to release a proton.
-
Substitution: The presence of electron-withdrawing substituents increases acidity, while electron-donating substituents decrease acidity. This is because electron-withdrawing groups stabilize the negative charge on the alkoxide ion, making it easier for the proton to be released.
-
Solvent Effects: The solvent used significantly impacts the acidity and basicity of alcohols. Protic solvents (solvents with O-H or N-H bonds) can stabilize both the alcohol and the alkoxide ion, influencing the equilibrium of the acid-base reaction.
Comparing Alcohols to Other Acids and Bases
Compared to strong acids like hydrochloric acid (HCl) or sulfuric acid (H₂SO₄), alcohols are significantly weaker acids. Similarly, compared to strong bases like sodium hydroxide (NaOH) or potassium hydroxide (KOH), alcohols are significantly weaker bases. Their amphoteric nature is only apparent in reactions with very strong acids or bases.
Practical Applications and Significance
The amphoteric nature of alcohols, although weak, plays a crucial role in various chemical reactions and processes:
-
Organic Synthesis: Alcohols are frequently used as solvents and reactants in organic synthesis due to their ability to act as both acids and bases under specific conditions. Their ability to participate in both acid-catalyzed and base-catalyzed reactions makes them versatile reagents.
-
Biological Systems: Alcohols are prevalent in biological systems. Their weak acidity and basicity contribute to their role in various biochemical processes. For instance, the hydroxyl groups in sugars and other biomolecules play crucial roles in hydrogen bonding and interactions with other molecules.
-
Industrial Applications: Alcohols find widespread applications in various industries, including pharmaceuticals, cosmetics, and food processing, due to their unique properties. Their ability to act as both acids and bases contributes to their usefulness in various applications.
Conclusion: Alcohols as Weak Brønsted Acids and Bases
In conclusion, alcohols exhibit amphoteric behavior according to the Brønsted-Lowry theory, acting as both weak acids and weak bases. Their ability to donate or accept a proton depends on the reaction conditions and the strength of the other reactant. The acidity of alcohols is primarily determined by the stability of the resulting alkoxide ion, influenced by factors such as inductive effects, resonance, and steric effects. Understanding the factors affecting alcohol's acid-base behavior is crucial in various chemical and biological contexts. While not as potent as strong acids or bases, their amphoteric nature contributes significantly to their versatility and widespread applications in numerous fields. Further research continues to unravel the complexities of alcohol's acid-base behavior, contributing to advancements in chemistry and related fields.
Latest Posts
Latest Posts
-
A First Course In Differential Equations
Mar 19, 2025
-
What Does Poly Mean Before A Chemical Compound
Mar 19, 2025
-
How Is The Half Life Of A Radioactive Parent Isotope Defined
Mar 19, 2025
-
What Elements Are Most Likley To Become Cations
Mar 19, 2025
-
What Is A Medium In Writing
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Alcohol A Acid And A Base Bronsted . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
