How Is The Half-life Of A Radioactive Parent Isotope Defined
Muz Play
Mar 19, 2025 · 6 min read
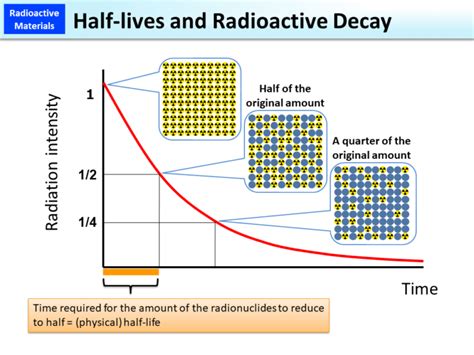
Table of Contents
How is the Half-Life of a Radioactive Parent Isotope Defined?
Radioactive decay is a fundamental process in nuclear physics, governing the transformation of unstable atomic nuclei into more stable ones. Understanding this process is crucial in various fields, from nuclear medicine and geology to archaeology and environmental science. A key concept in understanding radioactive decay is the half-life, a measure of how quickly a radioactive isotope decays. But what exactly is the half-life of a radioactive parent isotope, and how is it defined? Let's delve into the details.
Understanding Radioactive Decay
Before defining half-life, it's vital to grasp the basics of radioactive decay. Radioactive isotopes, or radionuclides, possess unstable nuclei. This instability arises from an imbalance in the number of protons and neutrons within the nucleus. To achieve stability, these nuclei undergo spontaneous transformations, emitting various particles and energy in the process. These emissions can include:
- Alpha particles (α): Two protons and two neutrons bound together, essentially a helium nucleus.
- Beta particles (β): High-energy electrons or positrons (anti-electrons).
- Gamma rays (γ): High-energy electromagnetic radiation.
This decay process is inherently random; we cannot predict precisely when a specific nucleus will decay. However, we can statistically predict the behavior of a large ensemble of nuclei. This statistical predictability forms the basis for defining the half-life.
Defining the Half-Life
The half-life of a radioactive parent isotope is defined as the time it takes for half of the initial number of parent nuclei in a sample to decay into its daughter isotope(s). It's crucial to understand that this is a statistical measure, not a prediction of individual nuclear decay. Imagine a large sample containing a trillion radioactive atoms; after one half-life, approximately 500 billion will have decayed.
It's not a fixed time for each nucleus. Some nuclei may decay very quickly, others much later. The half-life only describes the overall decay rate of the population. This is analogous to predicting the lifespan of a large group of people: you can't predict when a specific individual will die, but you can use statistical methods to predict the average lifespan of the group, and how many will have passed away after a certain period.
Mathematical Representation
The decay process follows first-order kinetics, meaning the decay rate is directly proportional to the number of undecayed nuclei present. This relationship is expressed mathematically as:
N(t) = N₀ * e^(-λt)
Where:
- N(t) is the number of undecayed nuclei at time t.
- N₀ is the initial number of nuclei at time t=0.
- λ is the decay constant (related to the probability of decay per unit time).
- e is the base of the natural logarithm (approximately 2.718).
- t is the time elapsed.
The half-life (t₁/₂) is related to the decay constant (λ) by the following equation:
t₁/₂ = ln(2) / λ ≈ 0.693 / λ
This equation shows the inverse relationship between the half-life and the decay constant. A larger decay constant means a shorter half-life, indicating faster decay. Conversely, a smaller decay constant implies a longer half-life, indicating slower decay.
Factors Affecting Half-Life
The half-life of a radioactive isotope is an intrinsic property, meaning it's determined by the specific nucleus and its structure. It is not affected by external factors such as:
- Temperature: Heating or cooling a radioactive sample will not alter its half-life.
- Pressure: Changes in pressure have no effect on the decay rate.
- Chemical state: The half-life of an element remains the same whether it is in elemental form, bound in a compound, or in a solution.
This independence from external factors makes half-life a valuable tool for various applications, as it remains constant regardless of environmental conditions.
Measuring Half-Life
Determining the half-life of a radioactive isotope involves measuring the activity of a sample over time. Activity refers to the rate of decay, typically measured in Becquerels (Bq), where 1 Bq represents one decay per second. By plotting the activity against time, a decay curve is obtained. The half-life can then be determined from this curve by finding the time it takes for the activity to reduce to half its initial value.
Sophisticated techniques are employed for accurate measurements, especially for isotopes with extremely short or long half-lives. These techniques often involve specialized detectors and data analysis methods.
Significance of Half-Life
The half-life of a radioactive isotope is a crucial parameter with wide-ranging applications:
Radioactive Dating:
- Carbon-14 dating: Used to determine the age of organic materials up to around 50,000 years old. The known half-life of Carbon-14 (5,730 years) allows scientists to estimate the time elapsed since the organism died based on the remaining Carbon-14 content.
- Uranium-lead dating: Used for dating rocks and minerals, providing insights into geological processes and the age of the Earth. The long half-lives of Uranium isotopes allow dating of materials billions of years old. Potassium-Argon dating is another example used for dating volcanic rocks.
Nuclear Medicine:
- Radioactive tracers: Radioisotopes with suitable half-lives are used in medical imaging and diagnostics. The half-life determines how long the tracer remains active in the body, affecting the imaging duration and radiation exposure.
- Radiotherapy: Radioisotopes with appropriate half-lives are used in cancer treatments, where the decay process releases energy to destroy cancerous cells. The half-life plays a role in the selection of suitable isotopes for targeted therapy.
Nuclear Engineering and Safety:
- Reactor design and operation: Half-lives of radioactive isotopes produced in nuclear reactors determine the long-term storage and disposal requirements of nuclear waste.
- Radiation safety: Understanding half-lives is essential for assessing and mitigating the risks associated with handling radioactive materials. The half-life determines how long a material remains radioactive and poses a potential hazard.
Environmental Science:
- Pollution monitoring: Radioactive tracers can be used to track pollutant movement in the environment. The half-life plays a role in determining the persistence of the pollutant.
Parent Isotope and Daughter Isotope
In radioactive decay, the original unstable nucleus is called the parent isotope. After decay, it transforms into a more stable nucleus, known as the daughter isotope. The half-life describes the rate of transformation from parent to daughter. For instance, in the decay of Carbon-14, the parent isotope is ¹⁴C, and the daughter isotope is ¹⁴N (Nitrogen-14). The decay chain may involve several steps, with each step having its own half-life.
Conclusion
The half-life of a radioactive parent isotope is a fundamental concept in nuclear physics and has profound implications across numerous scientific disciplines. Its precise definition, based on the statistical decay of a large number of nuclei, provides a robust and reliable measure of the decay rate, independent of external factors. Understanding half-life is crucial for various applications, from dating ancient artifacts to designing nuclear reactors and developing advanced medical treatments. Its significance continues to expand as our understanding of nuclear processes deepens and new technologies emerge.
Latest Posts
Latest Posts
-
Apogee And Perigee For An Elliptical Orbit
Mar 20, 2025
-
What Type Of Ions Do Bases Release
Mar 20, 2025
-
Where Does Transcription Take Place In Eukaryotic Cells
Mar 20, 2025
-
What Is A Horizontal Row In The Periodic Table Called
Mar 20, 2025
-
How To Identify Most Acidic Hydrogen
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Is The Half-life Of A Radioactive Parent Isotope Defined . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
